

Photoelectrocatalytic Urea Synthesis via co-Reduction of CO2 and $NO_{2}^{-}$ over Metallic Surface Modified TiO2
Received date: 2024-11-13
Online published: 2025-02-05
Supported by
National Natural Science Foundation of China(52300125); National Natural Science Foundation of China(22109004); Fundamental Research Funds for the Central Universities(BLX202259); Fundamental Research Funds for the Central Universities(BLX202257)
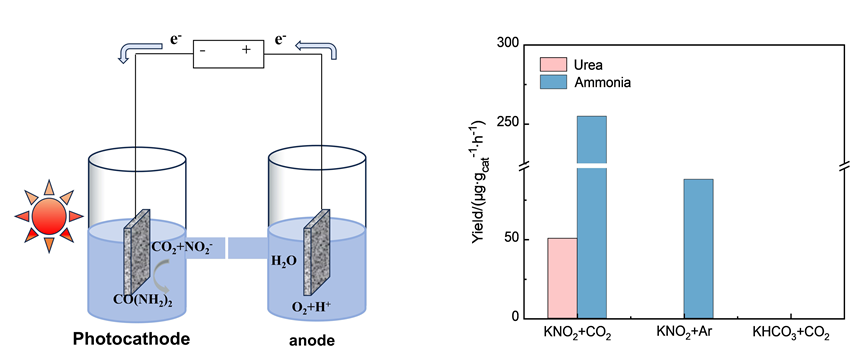
Using mild photoelectrocatalytic technology to co-reduce CO2 from the atmosphere and $NO_{2}^{-}$ from water pollutants into high-value-added chemical urea is an effective approach to achieving pollution reduction and carbon reduction goals. However, this reaction involves a 12-electron co-reduction with dual-substrate molecules, making it highly challenging with a complex pathway and high demands on the catalyst. So, there are few reports on this reaction currently. In this study, Ru, Cu, and Zn atom-modified TiO2 catalytic materials (Ru, Cu, and Zn-TiO2) were prepared via a hydrothermal method combined with photodeposition method. The specific synthesis processes are as follows: titanium butoxide was dropwise added into a H2O/HCl mixed solution. Then the solution and fluorine-doped tin oxide (FTO) substrate were transferred and sealed in a Teflon-lined stainless-steel autoclave and heated to 150 ℃ for 20 h. The obtained TiO2 photoelectrode was annealed in a muffle furnace at 350 ℃ for 2 h. The TiO2 with different metal atom surface modifications were prepared by adding metal salt solution and methanol in a beaker, placing the TiO2 photoelectrode in the solution and irradiating it with a 300 W xenon lamp for 1 h. We conducted catalytic urea synthesis experiments by using three-electrode configuration in a H-type quartz cell equipped with pretreated Nafion 117 membrane. The as-prepared semiconductor, Ag/AgCl electrode and Pt foil were utilized as the photocathode, reference and counter electrodes, respectively. 0.1 mol•L⁻1 KNO2 was used as the electrolyte in both the cathode and anode compartments. CO2 gas was injected into the cathode chamber during the photoelectrocatalytic test. The experimental results showed that Ru-TiO2 modified with 5% (w) Ru achieved a urea yield of 50.58 μmol•g⁻1•h⁻1 and a Faradaic efficiency (FE) of 16.66% under 2 h light irradiation at -0.1 V (vs. RHE). The 5% (w) Ru-TiO₂ catalyst exhibits a FE 1.65 times than that of TiO₂ under identical conditions. UV-Vis diffuse reflectance spectra (DRS) and electrochemical impedance spectra (EIS) indicate that Ru atoms can effectively enhance the light absorption, charge separation and transfer of TiO2. Photogenerated electrons transfer from TiO2 to Ru atomic sites, participating in the C—N coupling reaction. This work provides theoretical guidance for designing and synthesizing efficient catalysts for photoelectrocatalytic urea synthesis, offering novel idea and pathway for synergetic control of environmental pollution and carbon emissions.
Key words: titanium dioxide; photoelectrocatalysis; carbon dioxide; nitrite; urea
Yingqi Wang , Jiayi Chang , Min Li , Hong Liang , Zhiheng Li , Wenfu Xie , Qiang Wang . Photoelectrocatalytic Urea Synthesis via co-Reduction of CO2 and $NO_{2}^{-}$ over Metallic Surface Modified TiO2[J]. Acta Chimica Sinica, 2025 , 83(3) : 247 -255 . DOI: 10.6023/A24110345
| [1] | Dai, W.; Yu, J.; Luo, S.; Hu, X.; Yang, L.; Zhang, S.; Li, B.; Luo, X.; Zou, J. Chem. Eng. J. 2020, 389, 123430. |
| [2] | Liu, S.; Zhang, B.; Zhang, L.; Sun, J. Energy Chem. 2022, 71, 63. |
| [3] | Xu, Y. N.; Li, W.; Fu, H. Q.; Zhang, X. Y.; Zhao, J. Y.; Wu, X.; Yuan, H. Y.; Zhu, M.; Dai, S.; Liu, P. F.; Yang, H. G. Angew. Chem. Int. Ed. 2023, 62, e202217296. |
| [4] | Jiang, Y.-L.; Li, G.-C.; Chen, Q.-S.; Xu, Z.-N.; Ling, S.-S.; Guo, G.-C. Acta Chim. Sinica 2022, 80, 703 (in Chinese). |
| [4] | (蒋银龙, 李国超, 陈青松, 徐忠宁, 林姗姗, 郭国聪, 化学学报, 2022, 80, 703.) |
| [5] | Wang, X.-S.; Yang, X.; Chen, C.-H.; Li, H.-F.; Huang, Y.-B.; Cao, R. Acta Chim. Sinica 2022, 80, 22 (in Chinese). |
| [5] | (王旭生, 杨胥, 陈春辉, 李红芳, 黄远标, 曹荣, 化学学报, 2022, 80, 22.) |
| [6] | Zhu, X.; Zhou, X.; Jing, Y.; Li, Y. Nat. Commun. 2021, 12, 4080. |
| [7] | Geng, J.; Ji, S.; Jin, M.; Zhang, C.; Xu, M.; Wang, G.; Liang, C.; Zhang, H. Angew. Chem. Int. Ed. 2023, 62, e202210958. |
| [8] | Kempka, T.; Pl?tz, M.-L.; Schlüter, R.; Hamann, J.; Deowan, S. A.; Azzam, R. Energy Procedia 2011, 4, 2200. |
| [9] | Feng, Y.; Yang, H.; Zhang, Y.; Huang, X.; Li, L.; Cheng, T.; Shao, Q. Nano Lett. 2020, 20, 8282. |
| [10] | Zhang, R.; Hu, W.; Liu, J.; Xu, K.; Liu, Y.; Yao, Y.; Liu, M.; Zhang, X. G.; Li, H.; He, P.; Huo, S. Small 2024, 20, e2403285. |
| [11] | Zheng, J.; Xu, S.; Sun, J.; Zhang, J.; Sun, L.; Pan, X.; Li, L.; Zhao, G. Appl. Catal. B: Environ. 2023, 338, 123056. |
| [12] | Chen, Z.; Yang, J.; Yang, X.; Zhao, Y.; Kang, J.; Yang, F.; Zhang, Y.; Cheng, M.; Wang, G.; Duanmu, Q. Appl. Organomet. Chem. 2018, 32, e4356. |
| [13] | Yin, X.-W.; Fu, J.-J.; Zeng, M.; Liu, W.; Zhang, T.-Y.; Shen, P.-K.; Zhang, X.-Y. Acta Chim. Sinica 2022, 80, 503 (in Chinese). |
| [13] | (应霞薇, 浮建军, 曾敏, 刘文, 张天宇, 刘文, 沈培康, 张信义, 化学学报, 2022, 80, 503.) |
| [14] | Chen, S.; Lin, S.; Ding, L. X.; Wang, H. Small Methods 2023, 7, e2300003. |
| [15] | Guo, H.; Yuan, P.; Zhao, J.; Zhao, J.; Peng, Q.; Song, R. Chem. Eng. J. 2022, 450, 138198. |
| [16] | Liu, Y.; Qu, B.; Zhang, Z.; Sun, J.; Zhao, X.; Bai, L.; Jing, L. Mater. Res. Bull. 2022, 153, 111883. |
| [17] | Wang, R.; Yang, X.; Wang, Y.; Jia, J.; Wu, H. Mater. Today Commun. 2023, 35, 106125. |
| [18] | Yin, H.; Dong, F.; Wang, D.; Li, J. ACS Catal. 2022, 12, 14096. |
| [19] | Hong, D.; Lyu, L.-M.; Koga, K.; Shimoyama, Y.; Kon, Y. ACS Sustain. Chem. Eng. 2019, 7, 18955. |
| [20] | Du, J.; Huang, Y.; Huang, Z.; Wu, G.; Wu, B.; Han, X.; Chen, C.; Zheng, X.; Cui, P.; Wu, Y.; Jiang, J.; Hong, X. J. Am. Chem. Soc. 2022, 2, 1078. |
| [21] | Yoon, M.; Seo, M.; Jeong, C.; Jang, J. H.; Jeon, K. S. Chem. Mater. 2005, 17, 6069. |
/
| 〈 |
|
〉 |