

Self-Coupling Reactions of Photocatalyzed Dehydrogenated N-Alkoxyamide Compounds
Received date: 2025-01-06
Online published: 2025-03-07
Supported by
National Natural Science Foundation of China(22161046)
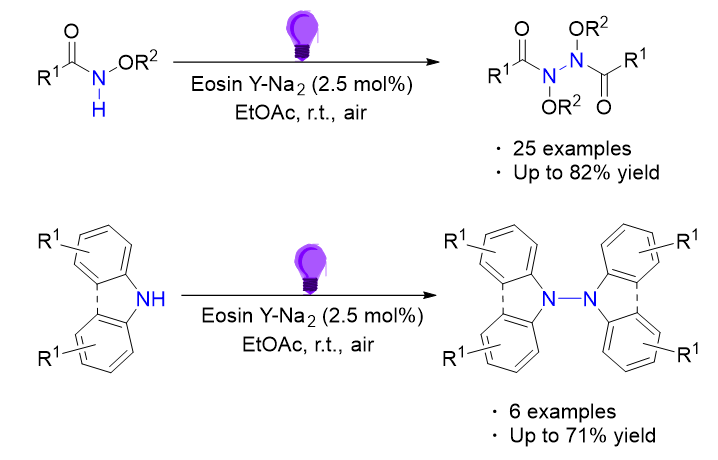
The N—N bond constitutes a fundamental structural motif in synthetic chemistry, ubiquitously present in organic compounds, natural products, and pharmaceutical agents. Beyond its structural significance, this bond demonstrates versatile functionalities in energy transfer systems, photoresponsive materials, and advanced dye technologies. Motivated by the critical importance of N—N bond formation, various synthetic methodologies have been explored. Among them, photocatalytic technology has stood out from numerous methods and become a highly regarded green organic synthesis means due to its mild conditions, environmental friendliness, and sustainable utilization. Based on this, this work reports the N—N self-coupling reaction of N-alkoxyamide and diarylamine compounds mediated by visible light. Employing commercially available Erythrosine Y disodium salt as the photosensitizer and ethyl acetate as the solvent, without metal catalysts, ligands and additives are required, a series of valuable functionalized hydrazines were provided at room temperature under air atmosphere with moderate to good yields. A total of 31 target compounds were synthesized, and the separation yield was up to 82%. Finally, the possible reaction mechanism was speculated through control experiments, hydrogen gas detection experiments, and relevant literature reports. This method not only features mild conditions and simple operation but also demonstrates good substrate universality, being applicable to various differently substituted benzene rings, aromatic heterocycles, and aliphatic amide substrates. Additionally, the target product can also be obtained with a separation yield of 49% under natural light conditions. It provides a simple, mild, and economical preparation approach for the synthesis of structurally complex hydrazine derivatives. The typical operational steps are as follows: In a 25 mL Schlenk tube, successively add N-alkoxyamide (0.2 mmol, 1 equiv.), Erythrosine Y disodium salt (2.5 mol%, 2.5% equiv.), and ethyl acetate (1 mL). Subject the reaction to irradiation at 10 W 413 nm LED for stirring and monitor the reaction progress using thin-layer chromatography (TLC). Once the starting materials are completely consumed, separate and purify the N—N self-coupling product using silica gel column chromatography.
Key words: no oxidant; diarylamine; green solvent; hydrazine compound
Dongxia Liu , Abuduwaili Kadierya , Qian Zhang , Jiajia Li , Yanqing Fan , Abudu Rexit Abulikemu . Self-Coupling Reactions of Photocatalyzed Dehydrogenated N-Alkoxyamide Compounds[J]. Acta Chimica Sinica, 2025 , 83(4) : 319 -325 . DOI: 10.6023/A25010008
| [1] | (a) Ramakumar, K.; Tunge, J. A. Chem. Commun. 2014, 50, 13056. |
| [1] | (b) Wang, H.; Jung, H.; Song, F.; Zhu, S.; Bai, Z.; Chen, D.; He, G.; Chang, S.; Chen, G. Nat. Chem. 2021, 13, 378. |
| [1] | (a) Blair, L. M.; Sperry, J. J. Nat. Prod. 2013, 76, 794. |
| [1] | (b) Rosen, B. R.; Werner, E. W.; O`Brien, A. G.; Baran, P. S. J. Am. Chem. Soc. 2014, 136, 5571. |
| [2] | (a) Gaulier, S. M.; McKay, R.; Swain, N. A. Tetrahedron Lett. 2011, 52, 6000. |
| [2] | (b) Zhou, C. H.; Wang, Y. Curr. Med. Chem. 2012, 19, 239. |
| [2] | (c) Kü?ükgüzel, ?. G.; ?enkarde?, S. Eur. J. Med. Chem. 2015, 97, 786. |
| [2] | (d) Li, Q. H.; Lin, Q.; Kim, H.; Yun, Z. Am. J. Cancer Res. 2017, 7, 1084. |
| [3] | (a) Beharry, A. A.; Woolley, G. A. Chem. Soc. Rev. 2011, 40, 4422. |
| [3] | (b) Sun, C. L.; Wang, C.; Boulatov, R. ChemPhotoChem 2019, 3, 268. |
| [3] | (c) Merino, E. Chem. Soc. Rev. 2011, 40, 3835. |
| [4] | (a) Perkin, W. H.; Tucker, S. H. J. Chem. Soc., rans. 1921, 119, 216. |
| [4] | (b) Pandit, P.; Yamamoto, K.; Nakamura, T.; Nakamura, K.; Kurashige, Y.; Yanai, T.; Nakamura, G.; Masaoka, S.; Furukawa, K.; Yakiyama, Y.; Kawano, M.; Higashibayashi, S. Chem. Sci. 2015, 6, 4160. |
| [5] | Branch, G. E. K.; Smith, J. F. J. Am. Chem. Soc. 1920, 42, 2405. |
| [6] | McLintock, J.; Tucker, S. H. J. Chem. Soc. 1927, 1214. |
| [7] | Yan, X. M.; Chen, Z. M.; Yang, F.; Huang, Z. Z. Synlett 2011, 2011, 569. |
| [8] | Ryan, M. C.; Martinelli, J. R.; Stahl, S. S. J. Am. Chem. Soc. 2018, 140, 9074. |
| [9] | Song, F. F.; Zhu, S. Y.; Wang, H.; Chen, G. Chin. J. Org. Chem. 2021, 41, 4050. (in Chinese) |
| [9] | (宋方方, 朱士阳, 王浩, 陈弓, 有机化学, 2021, 41, 4050.) |
| [10] | Zhu, S. Y.; He, W. J.; Shen, G. C.; Bai, Z. Q.; Song, F. F.; He, G.; Wang, H.; Chen, G. Angew. Chem. Int. Ed. 2024, 63, e202312465. |
| [11] | Feng, E. Q.; Hou, Z. W.; Xu, H. C. Chin. J. Org. Chem. 2019, 39, 1424. (in Chinese) |
| [11] | (冯恩祺, 侯中伟, 徐海超, 有机化学, 2019, 39, 1424.) |
| [12] | Nasier, A.; Chang, X. H.; Guo, C. J. Org. Chem. 2021, 86, 16068. |
| [13] | Luo, G. Z.; Han, Y.; Zhang, R. H.; Ding, D. M.; Dian, L. Y. Asian J. Org. Chem. 2022, 11, e202200553. |
| [14] | Balakrishna, B.; Mossin, S.; Kramer, S. Chem. Commun. 2022, 58, 10977. |
| [15] | Kadierya, A.; Reziguli, Y.; Li, J. J.; Luo, S. W.; Abulikemu, A. R. Acta Chim. Sinica 2024, 82, 731. (in Chinese) |
| [15] | (卡迪尔亚?阿布都外力,热孜古丽?玉努斯, 李佳佳, 罗时玮, 阿布都热西提?阿布力克木, 化学学报, 2024, 82, 731) |
| [16] | Ciamician, G. Science 1912, 36, 385. |
| [17] | (a) Tazuke, S.; Kitamura, N. J. Chem. Soc., hem. Commun. 1977, 15, 515. |
| [17] | (b) Dhara, A. K.; Maity, S.; Dhar, B. B. Org. Lett. 2021, 23, 3269. |
/
| 〈 |
|
〉 |