

C(sp3)—H Bond Oximinylation
Received date: 2025-01-02
Online published: 2025-03-13
Supported by
Science and Technology Major Program of Gansu Province of China(22ZD6FA006); National Natural Science Foundation of China(21871123); National Natural Science Foundation of China(22171120)
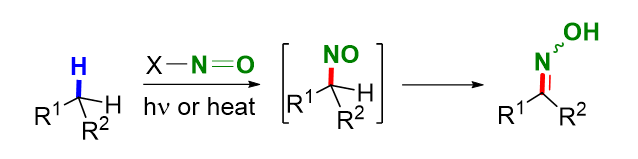
Oxime is a widely used organic compound with numerous applications in chemistry, biology, environmental science, medicine, pesticides, materials, and related industrial production. Among the various synthetic methods, directly converting carbon-hydrogen bonds in hydrocarbon compounds into oximes is the most straightforward and practical approach for synthesizing oximes. The oximinylations of reactive methylene and methyl C(sp3)—H bonds often involve oximinylating reagents such as nitrous acid, nitrosyl halides, nitrite, and nitrosyl esters, with the nitrosyl cation as a crucial intermediate. In contrast, radical-mediated oximinylations have a broader substrate scope and can even be applied to substrates containing only unactivated C(sp3)—H bonds. Mechanistically, the reactions involve the homolytic cleavage of a nitroso compound via light irradiation or heating, generating a heteroatom-centered radical and a nitric oxide radical. The heteroatom-centered radical subsequently abstracts a hydrogen atom from the substrate to form an alkyl radical, which then undergoes subsequent radical reactions and finally couples with the nitric oxide radical. This coupling produces a nitroso intermediate, which isomerizes to oxime. This paper reviews the oximinylations of saturated C(sp3)—H bonds from the early 20th century to the present. It categorizes the reactions based on their mechanisms, including the oximinylations of reactive methylene and methyl C(sp3)—H bonds, radical-mediated oximinylations of C(sp3)—H bonds, and metal-mediated oximinylations of C(sp3)—H bonds. Additionally, this paper not only elucidates the development of the C(sp3)—H oximinylation reaction but also discusses the challenges in this field and offers future perspectives with the aim of providing insights for innovative applications in synthetic chemistry and industrial processes.
Key words: C—H bond activation; oxime; oximinylation; nitric oxide; nitrosyl cation
Antuo Xu , Junyi Li , Qiang Liu . C(sp3)—H Bond Oximinylation[J]. Acta Chimica Sinica, 2025 , 83(4) : 390 -400 . DOI: 10.6023/A25010005
| [1] | Adams, J. P. J. Chem. Soc., 2000, 125. |
| [2] | Palacios, F.; de Retana, A. M. O.; de Marigorta, E. M.; de los Santos, J. M. Org. Prep. Proced. Int. 2002, 34, 219. |
| [3] | Kumar, R.; Chowdhury, B. Ind. Eng. Chem. Res. 2014, 53, 16587. |
| [4] | Bolotin, D. S.; Bokach, N. A.; Demakova, M. Y.; Kukushkin, V. Y. Chem. Rev. 2017, 117, 13039. |
| [5] | Bolotin, D. S.; Bokach, N. A.; Kukushkin, V. Y. Coord. Chem. Rev. 2016, 313, 62. |
| [6] | Ilinski, M.; Knorre, G. Ber. Dtsch. Chem. Ges. 1885, 18, 699. |
| [7] | Voloshin, Y. Z.; Novikov, V. V.; Nelyubina, Y. V. RSC Adv. 2015, 5, 72621. |
| [8] | Pan, T.-M.; Ye, J.-H.; Li, J.-N.; Gui, K.; Li, J.; Feng, J.-T.; Ma, Z.-Q.; Lei, P.; Gao, Y.-Q. J. Agric. Food. Chem. 2023, 71, 3164. |
| [9] | Ye, J.; Liu, X.; Zhou, R.; Hui, T.; Li, J.; Feng, J.; Ma, Z.-Q.; Gao, Y. Int. J. Food Microbiol. 2024, 409, 110461. |
| [10] | Balcerzak, L.; Surowiak, A. K.; Groborz, K.; Stró?ak, S.; Piekarska, K.; Strub, D. J. Toxicology 2023, 490, 153510. |
| [11] | Sekine, Y.JP 2004244408, 2004. |
| [12] | Komatsu, N.; Shimotomai, N.; Konosu, M. JP 62195309, 1987. |
| [13] | Oshchepkova, E. P.; Fridman, A. L.; Zalesov, V. S.; Kon'shina, L. O.; Kolobov, N. A.; Moiseev, I. K. SU 896140, 1982. |
| [14] | Tuloup, R.; Philippe, M.EP 1023894, 2000. |
| [15] | Chen, B.; Ning, Z.; Dong, Y. CN 112522718, 2021. |
| [16] | Cheng, Z.; Liu, Z.; Li, F.; Cao, W.; Zheng, Y.; Hu, Z. CN 110204272, 2019. |
| [17] | Worek, F.; Thiermann, H.; Wille, T. Chem. Biol. Interact. 2016, 259, 93. |
| [18] | Mercey, G.; Verdelet, T.; Renou, J.; Kliachyna, M.; Baati, R.; Nachon, F.; Jean, L.; Renard, P.-Y. Acc. Chem. Res. 2012, 45, 756. |
| [19] | Letendre, L.; Harriman, J.; Drag, M.; Mullins, A.; Malinski, T.; Rehbein, S. J. Vet. Pharmacol. Ther. 2017, 40, 35. |
| [20] | Nolan, T. J.; Lok, J. Curr. Pharm. Biotechnol. 2012, 13, 1078. |
| [21] | Bednarczyk-Cwynar, B.; Zaprutko, L. Phytochem. Rev. 2015, 14, 203. |
| [22] | Fylaktakidou, K. C.; Hadjipavlou-Litina, D. J.; Litinas, K. E.; Varella, E. A.; Nicolaides, D. N. Curr. Pharm. Des. 2008, 14, 1001. |
| [23] | Nikitjuka, A.; Jirgensons, A. Chem. Heterocycl. Compd. 2014, 49, 1544. |
| [24] | Lindell, S.; Dickhaut, J.; Jakobi, H.; Tiebes, J.; Jans, D.; Thoenessen, M.-T.; Waibel, J. M. EP 1236727, 2002. |
| [25] | Li, Q.; Cai, B.-G.; Li, L.; Xuan, J. Org. Lett. 2021, 23, 6951. |
| [26] | ābele, E.; Lukevics, E. J. H. Heterocycles 2000, 53, 2285. |
| [27] | Tan, Y.; Hartwig, J. F. J. Am. Chem. Soc. 2010, 132, 3676. |
| [28] | Lohse-Fraefel, N.; Carreira, E. M. Chem.-Eur. J. 2009, 15, 12065. |
| [29] | Ooi, T.; Takahashi, M.; Doda, K.; Maruoka, K. J. Am. Chem. Soc. 2002, 124, 7640. |
| [30] | Gagosz, F.; Zard, S. Z. Synlett 1999, 1999, 1978. |
| [31] | Nicastri, M. C.; Lehnherr, D.; Lam, Y.-H.; DiRocco, D. A.; Rovis, T. J. Am. Chem. Soc. 2020, 142, 987. |
| [32] | Benati, L.; Leardini, R.; Minozzi, M.; Nanni, D.; Scialpi, R.; Spagnolo, P.; Strazzari, S.; Zanardi, G. Angew. Chem., Int. Ed. 2004, 43, 3598. |
| [33] | Qi, X.-K.; Zheng, M.-J.; Yang, C.; Zhao, Y.; Guo, L.; Xia, W. J. Am. Chem. Soc. 2023, 145, 16630. |
| [34] | Zheng, Y.; Wang, Z.-J.; Ye, Z.-P.; Tang, K.; Xie, Z.-Z.; Xiao, J.-A.; Xiang, H.-Y.; Chen, K.; Chen, X.-Q.; Yang, H. Angew. Chem., Int. Ed. 2022, 61, e202212292. |
| [35] | Majhi, J.; Dhungana, R. K.; Rentería-Gómez, á.; Sharique, M.; Li, L.; Dong, W.; Gutierrez, O.; Molander, G. A. J. Am. Chem. Soc. 2022, 144, 15871. |
| [36] | Huang, T.; Liu, C.; Yuan, P.-F.; Wang, T.; Yang, B.; Ma, Y.; Liu, Q. Green Chem. 2024, 26, 9859. |
| [37] | Scheinbaum, M. L. J. Org. Chem. 1970, 35, 2785. |
| [38] | Prateeptongkum, S.; Jovel, I.; Jackstell, R.; Vogl, N.; Weckbecker, C.; Beller, M. Chem. Commun. 2009, 1990. |
| [39] | Yuan, P.-F.; Huang, T.; He, J.; Huang, X.-T.; Jin, X.-L.; Sun, C.-L.; Wu, L.-Z.; Liu, Q. Org. Chem. Front. 2021, 8, 5785. |
| [40] | Zheng, D.; Pl?ger, S.; Daniliuc, C. G.; Studer, A. Angew. Chem., Int. Ed. 2021, 60, 8547. |
| [41] | Wang, Z.; Wierich, N.; Zhang, J.; Daniliuc, C. G.; Studer, A. J. Am. Chem. Soc. 2023, 145, 8770. |
| [42] | Sang, J.-W.; Chen, H.; Zhang, Y.; Wang, J.; Zhang, W.-D. Green Chem. 2024, 26, 7849. |
| [43] | Yuan, P.-F.; Huang, X.-T.; Long, L.; Huang, T.; Sun, C.-L.; Yu, W.; Wu, L.-Z.; Chen, H.; Liu, Q. 2024, 63, e202317968. |
| [44] | Li, W.; Diao, C.-C.; Lu, Y.-L.; Li, H.-F. Org. Lett. 2024, 26, 6253. |
| [45] | Li, W.; Zhao, L.-T.; Diao, C.-C.; Zhang, G.-H.; Li, H.-F. Org. Lett. 2025, 27, 252. |
| [46] | Lan, J.-Y.; Li, X.-L.; Xu, M.-Y.; Zhang, B.; Luo, J.; Zhou, Y.; Wang, T. J. Org. Chem. 2025, 90, 250. |
| [47] | Yang, H.-X.; Li, M.-M.; Zhang, A.-J.; Guo, J.-F.; Yu, Y.-Q.; Ding, W. Chin. Chem. Lett. 2025, 36, 110425. |
| [48] | Chen, Z.-L.; Li, Q.-Q.; Studer, A.; Xuan, J. Sci. China: Chem. 2025, 68, 118. |
| [49] | Sang, J.-W.; Zhang, Y.; Xia, D.-D.; Hu, Z.-M.; Wang, J.-X.; Zhang, W.-D. Org. Chem. Front. 2025, 12, 869. |
| [50] | Aston, J. G.; Menard, D. F.; Mayberry, M. G. J. Am. Chem. Soc. 1932, 54, 1530. |
| [51] | Aston, J. G.; Mayberry, M. G. J. Am. Chem. Soc. 1935, 57, 1888. |
| [52] | Bennett, G. B.; Mason, R. B.; Alden, L. J.; Roach, J. B. Jr. J. Med. Chem. 1978, 21, 623. |
| [53] | Kataoka, M.; Ohno, M. Bull. Chem. Soc. Jpn. 1973, 46, 3474. |
| [54] | Taylor, E. C.; Dumas, D. J. J. Org. Chem. 1980, 45, 2485. |
| [55] | Rogic, M. M.; Vitrone, J.; Swerdloff, M. D. J. Am. Chem. Soc. 1977, 99, 1156. |
| [56] | Leis, J. R.; Pe?a, M. E.; Williams, D. L. H. J. Chem. Soc., Chem. Commun. 1987, 45. |
| [57] | Lee, S.; Fuchs, P. L. Can. J. Chem. 2006, 84, 1442. |
| [58] | Gao, X.; Zhang, F.; Deng, G.; Yang, L. Org. Lett. 2014, 16, 3664. |
| [59] | Tokuyama, H.; Cho, H.; Iwama, Y.; Noro, T.; Okano, K. Heterocycles 2014, 88, 1433. |
| [60] | Claisen, L.; Manasse, O. Ber. Dtsch. Chem. Ges. 1889, 22, 526. |
| [61] | Lynn, E. V. J. Am. Chem. Soc. 1919, 41, 368. |
| [62] | Lynn, E. V.; Arkley, H. L. J. Am. Chem. Soc. 1923, 45, 1045. |
| [63] | Mitchell, S.; Carson, S. J. Chem. Soc. 1936, 1005. |
| [64] | Naylor, M. A.; Anderson, A. W. J. Org. Chem. 1953, 18, 115. |
| [65] | Müller, E.; Metzger, H. Chem. Ber. 1954, 87, 1282. |
| [66] | Mackor, A.; Veenland, J. U.; de Boer, T. J. Recl. Trav. Chim. Pays-Bas 1969, 88, 1249. |
| [67] | Mackor, A.; de Boer, T. J. Recl. Trav. Chim. Pays-Bas 1970, 89, 151. |
| [68] | Mackor, A.; de Boer, T. J. Recl. Trav. Chim. Pays-Bas 1970, 89, 159. |
| [69] | Mackor, A.; de Boer, T. J. Recl. Trav. Chim. Pays-Bas 1970, 89, 164. |
| [70] | Müller, E.; B?ttcher, A. E. Tetrahedron Lett. 1970, 11, 3083. |
| [71] | Hirabayashi, T.; Sakaguchi, S.; Ishii, Y. Angew. Chem., Int. Ed. 2004, 43, 1120. |
| [72] | Hashimoto, M.; Sakaguchi, S.; Ishii, Y. Chem.-Asian J. 2006, 1, 712. |
| [73] | Wei, W.-T.; Zhu, W.-M.; Ying, W.-W.; Wu, Y.; Huang, Y.-L.; Liang, H. Org. Biomol. Chem. 2017, 15, 5254. |
| [74] | Wysocki, J.; Teles, J. H.; Dehn, R.; Trapp, O.; Sch?fer, B.; Schaub, T. ChemPhotoChem 2018, 2, 22. |
| [75] | Lebl, R.; Cantillo, D.; Kappe, C. O. React. Chem. Eng. 2019, 4, 738. |
| [76] | Griffiths, O. M.; Ruggeri, M.; Baxendale, I. R. Synlett 2020, 31, 1907. |
| [77] | Barton, D. H. R.; Hesse, R. H.; Pechet, M. M.; Smith, L. C. J. Chem. Soc., 1979, 1159. |
| [78] | Barton, D. H. R.; Beaton, J. M.; Geller, L. E.; Pechet, M. M. J. Am. Chem. Soc. 1961, 83, 4076. |
| [79] | Barton, D. H. R.; Lier, E. F.; McGhie, J. F. J. Chem. Soc. C 1968, 1031. |
| [80] | Suginome, H.; Kojima, T.; Orito, K.; Masamune, T. Tetrahedron 1971, 27, 291. |
| [81] | Corey, E. J.; Arnett, J. F.; Widiger, G. N. J. Am. Chem. Soc. 1975, 97, 430. |
| [82] | Ishmuratov, G. Y.; Kharisov, R. Y.; Shayakhmetova, A. K.; Botsman, L. P.; Shitikova, O. V.; Tolstikov, G. A. Chem. Nat. Compd. 2005, 41, 643. |
| [83] | Huang, T.; Yuan, P.-F.; Dong, K.; Zong, Y.-Y.; Liu, C.; Wang, R.-H.; Jin, X-L.; Liu, Q. Org. Chem. Front. 2023, 10, 4559. |
| [84] | Makarycheva-Mikhailova, A. V.; Gushchin, P. V.; Kopylovich, M. N.; Ganebnykh, I. N.; Charushin, V. N.; Haukka, M.; Pombeiro, A. J. L.; Kukushkin, V. Y. Inorg. Chem. Commun. 2006, 9, 869. |
| [85] | Yu, J.-T.; Lu, M. Org. Biomol. Chem. 2015, 13, 7397. |
/
| 〈 |
|
〉 |