

Two Step Synthesis of Octreotide-doxorubicin Conjugates Based on Disulfide Bond Linker
Received date: 2025-02-02
Online published: 2025-04-08
Supported by
National Natural Science Foundation of China(22177058); Taishan Scholar Project of Shandong Province(tsqn202312168); Natural Science Foundation of Shandong Province(ZR2024YQ061)
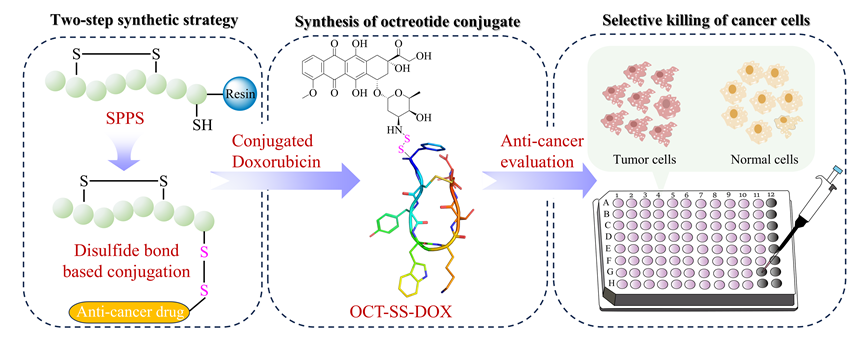
Cancer is still a global health challenge and developing novel anticancer drugs with new skeletons is of great importance. Recently, peptide-based agents have become promising candidates for anticancer treatments, especially for the treatment of drug-resistant refractory malignancies. Peptide drug conjugates (PDCs) are novel targeted anticancer drugs with advantages including high permeability to cancer tissues, low production costs, and ease of structural optimization. PDCs could significantly enhance the selectivity and anticancer activity of cytotoxic drugs. Octreotide, which is a cyclic peptide inherently containing one pair of disulfide bonds, could specifically target the somatostatin receptor 2 (SSTR2) on the surface of cancer cells, leading to its widespread clinical applications. The reducible disulfide bonds serve as a commonly utilized cleavable linker in PDCs. However, traditional synthesis of octreotide-drug conjugates based on disulfide bond linkers generally used the “three-step” strategy, including complex synthetic procedure and inducing low isolation yields. To address this issue, we developed a “two-step” synthetic strategy. First, octreotide containing one disulfide bond and the free thiol group (OCT-SH) was synthesized by the straightforward solid-phase peptide synthesis (SPPS). Subsequently, the OCT-SH was conjugated to small molecule drugs through site-specific liquid-phase reaction. To achieve the efficient and economic synthesis of OCT-SH, various SPPS based strategies and peptide cleavage systems were explored. Considering the chromatographic purity and synthetic yield, the optimal synthetic route (SPPS-2) and peptide cleavage system were established. To further validate the robustness of SPPS-2, the influences of different solid resins and peptide cleavage time on synthetic efficiency were investigated. After the efficient manual synthesis of OCT-SH, the promising chemotherapeutic drug doxorubicin (DOX) was covalently coupled to OCT-SH through liquid-phase reaction, affording the octreotide-doxorubicin conjugate. In vitro anticancer studies indicated that the octreotide-doxorubicin conjugate exhibited potent anticancer activity, significantly reducing toxicity to normal cells while achieving selectivity towards cancer cells. The cellular uptake experiment indicated that the covalent conjugation of doxorubicin did not significantly impair the cellular uptake potency of octreotide by cancer cells. Collectively, this study established the “two-step” synthetic strategy, enabling efficient and straightforward preparation of octreotide-doxorubicin conjugates containing the disulfide bond linker. This work also provides valuable references for the SPPS based synthesis of PDCs containing multiple disulfide bonds and inspires the development of novel PDCs for cancer therapy.
Qianyao Yu , Ming Meng , Jingfang Yao , Shanshan Du , Yunkun Qi . Two Step Synthesis of Octreotide-doxorubicin Conjugates Based on Disulfide Bond Linker[J]. Acta Chimica Sinica, 2025 , 83(4) : 341 -353 . DOI: 10.6023/A25020034
| [1] | Siegel, R. L.; Giaquinto, A. N.; Jemal, A. CA-Cancer J. Clin. 2024, 74, 12. |
| [2] | Anderson, R. L.; Balasas, T.; Callaghan, J.; Coombes, R. C.; Evans, J.; Hall, J. A.; Kinrade, S.; Jones, D.; Jones, P. S.; Jones, R.; Marshall, J. F.; Panico, M. B.; Shaw, J. A.; Steeg, P. S.; Sullivan, M.; Tong, W.; Westwell, A. D.; Ritchie, J. W. A.; Berg, R.; Drysdale, M.; Eccles, S.; Elvin, P.; Harris, A.; Ireson, C.; Machesky, L.; McLeod, R.; Muschel, R.; Newell, H.; Pittman, M.; Roman, B.; Santos, C.; Sibson, N.; Smith, A.; Waddell, I. Nat. Rev. Clin. Oncol. 2019, 16, 185. |
| [3] | Ma, Q. X.; Xu, Q. Q.; Zhao, J. J.; Zhang, W. W.; Wang, Q.; Fang, J.; Lu, Z. M.; Liu, J.; Ma, L. N. Cancer Lett. 2021, 520, 243. |
| [4] | Bargh, J. D.; Isidro-Llobet, A.; Parker, J. S.; Spring, D. R. Chem. Soc. Rev. 2019, 48, 4361. |
| [5] | Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Signal Transduct. Target. Ther. 2022, 7, 93. |
| [6] | Paul, S.; Konig, M. F.; Pardoll, D. M.; Bettegowda, C.; Papadopoulos, N.; Wright, K. M.; Gabelli, S. B.; Ho, M.; van Elsas, A.; Zhou, S. Nat. Rev. Cancer 2024, 24, 399. |
| [7] | Fu, C.; Yu, L.; Miao, Y.; Liu, X.; Yu, Z.; Wei, M. Acta Pharm. Sin. B 2023, 13, 498. |
| [8] | Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Int. J. Oncol. 2020, 57, 678. |
| [9] | Luan, X.; Wu, Y.; Shen, Y.-W.; Zhang, H.; Zhou, Y.-D.; Chen, H.-Z.; Nagle, D. G.; Zhang, W.-D. Nat. Prod. Rep. 2021, 38, 7. |
| [10] | Cooper, B. M.; Iegre, J.; O' Donovan, D. H.; Halvarsson, M. O.; Spring, D. R. Chem. Soc. Rev. 2021, 50, 1480. |
| [11] | Dean, T. T.; Jelu-Reyes, J.; Allen, A. C.; Moore, T. W. J. Med. Chem. 2024, 67, 1641. |
| [12] | He, H.; Deng, X.; Wang, Z.; Chen, J. Eur. J. Med. Chem. 2025, 284, 117204. |
| [13] | Sagar, B.; Gupta, S.; Verma, S. K.; Reddy, Y. V. M.; Shukla, S. Eur. J. Med. Chem. 2025, 283, 117131. |
| [14] | Zhang, P.; Jian, C.; Jian, S.; Zhang, Q.; Sun, X.; Nie, L.; Liu, B.; Li, F.; Li, J.; Liu, M.; Liang, S.; Zeng, Y.; Liu, Z. J. Med. Chem. 2019, 62, 11108. |
| [15] | Vitale, I.; Yamazaki, T.; Wennerberg, E.; Sveinbjornsson, B.; Rekdal, O.; Demaria, S.; Galluzzi, L. Trends Cancer 2021, 7, 557. |
| [16] | Yin, H.; Chen, X.-T.; Chi, Q.-N.; Ma, Y.-N.; Fu, X.-Y.; Du, S.-S.; Qi, Y.-K.; Wang, K.-W. Acta Pharmacol. Sin. 2023, 44, 201. |
| [17] | Yin, H.; Fu, X.-Y.; Gao, H.-Y.; Ma, Y.-N.; Yao, J.-F.; Du, S.-S.; Qi, Y.-K.; Wang, K.-W. Bioorg. Chem. 2023, 138, 106674. |
| [18] | Fu, X.-Y.; Yin, H.; Chen, X.-T.; Yao, J.-F.; Ma, Y.-N.; Song, M.; Xu, H.; Yu, Q.-Y.; Du, S.-S.; Qi, Y.-K.; Wang, K.-W. J. Med. Chem. 2024, 67, 3885. |
| [19] | Chang, H.-N.; Liu, B.-Y.; Qi, Y.-K.; Zhou, Y.; Chen, Y.-P.; Pan, K.-M.; Li, W.-W.; Zhou, X.-M.; Ma, W.-W.; Fu, C.-Y.; Qi, Y.-M.; Liu, L.; Gao, Y.-F. Angew. Chem. Int. Ed. 2015, 54, 11760. |
| [20] | Zhou, X.; Zuo, C.; Li, W.; Shi, W.; Zhou, X.; Wang, H.; Chen, S.; Du, J.; Chen, G.; Zhai, W.; Zhao, W.; Wu, Y.; Qi, Y.; Liu, L.; Gao, Y. Angew. Chem. Int. Ed. 2020, 59, 15114. |
| [21] | Qi, Y. K.; Zheng, J. S.; Liu, L. Chem 2024, 10, 2390. |
| [22] | Qian, Y.; Sun, Y.; Shi, P.; Zhou, X.; Zhang, Q.; Dong, Q.; Jin, S.; Qiu, L.; Niu, X.; Zhou, X.; Zhao, W.; Wu, Y.; Zhai, W.; Gao, Y. Acta Pharm. Sin. B 2024, 14, 1150. |
| [23] | Vrettos, E. I.; Mezo, G.; Tzakos, A. G. Beilstein J. Org. Chem. 2018, 14, 930. |
| [24] | Alas, M.; Saghaeidehkordi, A.; Kaur, K. J. Med. Chem. 2021, 64, 216. |
| [25] | Lindberg, J.; Nilvebrant, J.; Nygren, P.-A.; Lehmann, F. Molecules 2021, 26, 6042. |
| [26] | Li, Y.-J.; Fang, C.-B.; Wang, S.-S.; Chen, X.-Q.; Li, Y.; Liu, Q.; Qi, Y.-K.; Du, S.-S. Bioorgan. Med. Chem. 2024, 111, 117869. |
| [27] | Gong, L.; Zhao, H.; Liu, Y.; Wu, H.; Liu, C.; Chang, S.; Chen, L.; Jin, M.; Wang, Q.; Gao, Z.; Huang, W. Acta Pharm. Sin. B 2023, 13, 3659. |
| [28] | Hennrich, U.; Kopka, K. Pharmaceuticals 2019, 12, 114. |
| [29] | Strosberg, J. R.; Caplin, M. E.; Kunz, P. L.; Ruszniewski, P. B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E. M.; Yao, J. C.; Pavel, M. E.; Grande, E.; Van Cutsem, E.; Seregni, E.; Duarte, H.; Gericke, G.; Bartalotta, A.; Mariani, M. F.; Demange, A.; Mutevelic, S.; Krenning, E. P.; Investigators, N.-E.-. Lancet Oncol. 2021, 22, 1752. |
| [30] | Griffiths, G. L.; Vasquez, C.; Escorcia, F.; Clanton, J.; Lindenberg, L.; Mena, E.; Choyke, P. L. Adv. Drug Deliv. Rev. 2022, 181, 114086. |
| [31] | Huang, C. M.; Wu, Y. T.; Chen, S. T. Chem. Biol. 2000, 7, 453. |
| [32] | Gaviglio, L.; Gross, A.; Metzler-Nolte, N.; Ravera, M. Metallomics 2012, 4, 260. |
| [33] | Lelle, M.; Kaloyanova, S.; Freidel, C.; Theodoropoulou, M.; Musheev, M.; Niehrs, C.; Stalla, G.; Peneva, K. Mol. Pharm. 2015, 12, 4290. |
| [34] | Ebaston, T. M.; Rozoysky, A.; Zaporozhets, A.; Bazyleyich, A.; Tuchinsky, H.; Marks, V.; Gellerman, G.; Patsenker, L. D. ChemMedChem 2019, 14, 1727. |
| [35] | Rozovsky, A.; Ebaston, T. M.; Zaporozhets, A.; Bazylevich, A.; Tuchinsky, H.; Patsenker, L.; Gellerman, G. RSC Adv. 2019, 9, 32656. |
| [36] | Wang, M.; Liu, J.; Xia, M.; Yin, L.; Zhang, L.; Liu, X.; Cheng, Y. Eur. J. Med. Chem. 2024, 265, 116119. |
| [37] | Zhou, J.; Li, Y.; Huang, W.; Shi, W.; Qian, H. Eur. J. Med. Chem. 2021, 224, 113712. |
| [38] | Deng, X.; Mai, R. Y.; Zhang, C. Y.; Yu, D. B.; Ren, Y. C.; Li, G.; Cheng, B. B.; Li, L.; Yu, Z. Q.; Chen, J. J. Eur. J. Med. Chem. 2021, 213, 113050. |
| [39] | Sharma, A.; Singh, L. R. Eur. J. Med. Chem. 2024, 271, 116456. |
| [40] | Qin, F.; Zhou, H.; Li, J.; Liu, J.; Wang, Y.; Bai, R.; Liu, S.; Manman, M.; Liu, T.; Gao, F.; Du, P.; Lu, X.; Chen, C. Eur. J. Pharmacol. 2021, 905, 174187. |
| [41] | Ding, J.; Wang, T.; Rong, Y.; He, C.; Chen, X. Chinese J. Chem. 2024, 42, 2957. |
| [42] | Huang, Y.; Luo, J.; Li, J.; Zhang, R.; Liu, X.; Fan, Q.; Huang, W. Acta Chim. Sinica 2024, 82, 903. (in Chinese) |
| [42] | (黄艳琴, 罗集文, 李佳启, 张瑞, 刘兴奋, 范曲立, 黄维, 化学学报, 2024, 82, 903.) |
| [43] | Li, M.; Zhang, J.; Liu, L.; Xu, S.; Liu, H. Acta Chim. Sinica 2024, 82, 856. (in Chinese) |
| [43] | (李梦丽, 张婕, 刘丽珍, 徐首红, 刘洪来, 化学学报, 2024, 82, 856.) |
| [44] | Beck, A.; Goetsch, L.; Dumontet, C.; Corva?a, N. Nat. Rev. Drug Discov. 2017, 16, 315. |
| [45] | Liang, Y.; Li, S.; Wang, X.; Zhang, Y.; Sun, Y.; Wang, Y.; Wang, X.; He, B.; Dai, W.; Zhang, H.; Wang, X.; Zhang, Q. J. Control. Release 2018, 275, 129. |
| [46] | White, B. H.; Whalen, K.; Kriksciukaite, K.; Alargova, R.; Yeung, T. A.; Bazinet, P.; Brockman, A.; DuPont, M.; Oller, H.; Lemelin, C.-A.; Soo, P. L.; Moreau, B.; Perino, S.; Quinn, J. M.; Sharma, G.; Shinde, R.; Sweryda-Krawiec, B.; Wooster, R.; Bilodeau, M. T. J. Med. Chem. 2019, 62, 2708. |
| [47] | Kciuk, M.; Gielecinska, A.; Mujwar, S.; Kolat, D.; Kaluzinska-Kolat, Z.; Celik, I.; Kontek, R. Cells 2023, 12, 659. |
| [48] | Lee, J.; Choi, M. K.; Song, I. S. Pharmaceuticals 2023, 16, 802. |
| [49] | Mattioli, R.; Ilari, A.; Colotti, B.; Mosca, L.; Fazi, F.; Colotti, G. Mol. Asp. Med. 2023, 93, 101205. |
| [50] | Wang, S.; Sun, L.; Cao, H.; Zhong, Y.; Shao, Z. Acta Chim. Sinica 2021, 79, 1023. (in Chinese) |
| [50] | (王苏杭, 孙灵娜, 曹涵, 钟一鸣, 邵正中, 化学学报, 2021, 79, 1023.) |
| [51] | Guillier, F.; Orain, D.; Bradley, M. Chem. Rev. 2000, 100, 2091. |
| [52] | Isidro-Llobet, A.; Alvarez, M.; Albericio, F. Chem. Rev. 2009, 109, 2455. |
| [53] | Harris, P. W. R.; Kowalczyk, R.; Yang, S.-H.; Williams, G. M.; Brimble, M. A. J. Pept. Sci. 2014, 20, 186. |
| [54] | Skalska, J.; Andrade, V. M.; Cena, G. L.; Harvey, P. J.; Gaspar, D.; Mello, é.; Henriques, S. T.; Valle, J.; Gomes, V. M.; Concei?ao, K.; Castanho, M.; Andreu, D. J. Med. Chem. 2020, 63, 9391. |
| [55] | Wang, J. Y.; Dong, L. Y.; Liu, Y. N.; Chen, X. T.; Ma, Y. N.; Yin, H.; Du, S. S.; Qi, Y. K.; Wang, K. W. Chin. J. Org. Chem. 2021, 41, 2800. (in Chinese) |
| [55] | (王金艳, 董黎颖, 刘雅妮, 陈西同, 马艳楠, 尹昊, 杜姗姗, 齐昀坤, 王克威, 有机化学, 2021, 41, 2800.) |
| [56] | Chen, X.-T.; Wang, J.-Y.; Ma, Y.-N.; Dong, L.-Y.; Jia, S.-X.; Yin, H.; Fu, X.-Y.; Du, S.-S.; Qi, Y.-K.; Wang, K. J. Pept. Sci. 2022, 28, e3368. |
| [57] | Ma, Y.; Liu, Y. N.; Wang, J.; Chen, X.; Yin, H.; Chi, Q.; Jia, S.; Du, S.; Qi, Y.; Wang, K. Chin. J. Org. Chem. 2022, 42, 498. (in Chinese) |
| [57] | (马艳楠, 刘雅妮, 王金艳, 陈西同, 尹昊, 迟巧娜, 贾世玺, 杜姗姗, 齐昀坤, 王克威, 有机化学, 2022, 42, 498.) |
| [58] | Yin, H.; Chen, X.; Fu, X.; Ma, Y.; Xu, Y.; Zhang, T.; Liang, S.; Du, S.; Qi, Y.; Wang, K. Acta Chim. Sinica 2022, 80, 444. (in Chinese) |
| [58] | (尹昊, 陈西同, 付邢言, 马艳楠, 徐以梅, 张特, 梁帅, 杜姗姗, 齐昀坤, 王克威, 化学学报, 2022, 80, 444.) |
| [59] | Song, M.; Liu, Q.; Yao, J.-F.; Wang, Y.-T.; Ma, Y.-N.; Xu, H.; Yu, Q.-Y.; Li, Z.; Du, S.-S.; Qi, Y.-K. Bioorgan. Med. Chem. 2024, 107, 117760. |
| [60] | Spears, R. J.; McMahon, C.; Chudasama, V. Chem. Soc. Rev. 2021, 50, 11098. |
| [61] | Pan, M.; Gao, S.; Zheng, Y.; Tan, X.; Lan, H.; Tan, X.; Sun, D.; Lu, L.; Wang, T.; Zheng, Q.; Huang, Y.; Wang, J.; Liu, L. J. Am. Chem. Soc. 2016, 138, 7429. |
| [62] | Zuo, C.; Shi, W.-W.; Chen, X.-X.; Glatz, M.; Riedl, B.; Flamme, I.; Pook, E.; Wang, J.; Fang, G.-M.; Bierer, D.; Liu, L. Sci. China: Chem. 2019, 62, 1371. |
| [63] | Liang, L.-J.; Chu, G.-C.; Qu, Q.; Zuo, C.; Mao, J.; Zheng, Q.; Chen, J.; Meng, X.; Jing, Y.; Deng, H.; Li, Y.-M.; Liu, L. Angew. Chem. Int. Ed. 2021, 60, 17171. |
| [64] | Ai, H.; Chu, G.-C.; Gong, Q.; Tong, Z.-B.; Deng, Z.; Liu, X.; Yang, F.; Xu, Z.; Li, J.-B.; Tian, C.; Liu, L. J. Am. Chem. Soc. 2022, 144, 18329. |
| [65] | Ai, H.; Sun, M.; Liu, A.; Sun, Z.; Liu, T.; Cao, L.; Liang, L.; Qu, Q.; Li, Z.; Deng, Z.; Tong, Z.; Chu, G.; Tian, X.; Deng, H.; Zhao, S.; Li, J.-B.; Lou, Z.; Liu, L. Nat. Chem. Biol. 2022, 18, 972. |
| [66] | Zhang, B.; Zheng, Y.; Chu, G.; Deng, X.; Wang, T.; Shi, W.; Zhou, Y.; Tang, S.; Zheng, J.-S.; Liu, L. Angew. Chem. Int. Ed. 2023, 62, e202306270. |
| [67] | Zhao, R.; Shi, P.; Cui, J.-b.; Shi, C.; Wei, X.-X.; Luo, J.; Xia, Z.; Shi, W.-W.; Zhou, Y.; Tang, J.; Tian, C.; Meininghaus, M.; Bierer, D.; Shi, J.; Li, Y.-M.; Liu, L. Angew. Chem. Int. Ed. 2023, 62, e202216365. |
| [68] | You, Y.; Xu, Z.; Chen, Y. Drug Deliv. 2018, 25, 448. |
| [69] | Randelovic, I.; Schuster, S.; Kapuvari, B.; Fossati, G.; Steinkuehler, C.; Mezo, G.; Tovari, J. Int. J. Mol. Sci. 2019, 20, 4763. |
| [70] | He, Y.; Yuan, X. M.; Lei, P.; Wu, S.; Xing, W.; Lan, X. L.; Zhu, H. F.; Huang, T.; Wang, G. B.; An, R.; Zhang, Y. X.; Shen, G. X. Acta Pharmacol. Sin. 2009, 30, 1053. |
| [71] | Zhang, J.; Jin, W.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Mol. Pharm. 2010, 7, 1159. |
| [72] | Sanwick, A. M.; Haugh, K. N.; Williams, E. J.; Perry, K. A.; Thiele, N. A.; Chaple, I. F. EJNMMI Radiopharm. Chem. 2024, 9, 88. |
| [73] | Zhou, Y.; Liu, X.; Xue, J.; Liu, L.; Liang, T.; Li, W.; Yang, X.; Hou, X.; Fang, H. J. Med. Chem. 2020, 63, 4701. |
| [74] | Lu, M.; Xing, H.; Shao, W.; Wu, P.; Fan, Y.; He, H.; Barth, S.; Zheng, A.; Liang, X.-J.; Huang, Y. Acta Pharm. Sin. B 2023, 13, 3945. |
| [75] | Fang, G.-M.; Li, Y.-M.; Shen, F.; Huang, Y.-C.; Li, J.-B.; Lin, Y.; Cui, H.-K.; Liu, L. Angew. Chem. Int. Ed. 2011, 50, 7645. |
| [76] | Fang, G.-M.; Wang, J.-X.; Liu, L. Angew. Chem. Int. Ed. 2012, 51, 10347. |
| [77] | Zheng, J.-S.; Tang, S.; Qi, Y.-K.; Wang, Z.-P.; Liu, L. Nat. Protoc. 2013, 8, 2483. |
| [78] | Dong, S.; Zheng, J.-S.; Li, Y.; Wang, H.; Chen, G.; Chen, Y.; Fang, G.; Guo, J.; He, C.; Hu, H.; Li, X.; Li, Y.; Li, Z.; Pan, M.; Tang, S.; Tian, C.; Wang, P.; Wu, B.; Wu, C.; Zhao, J.; Liu, L. Sci. China: Chem. 2024, 67, 1060. |
| [79] | Chi, Q.-N.; Jia, S.-X.; Yin, H.; Wang, L.-E.; Fu, X.-Y.; Ma, Y.-N.; Sun, M.-P.; Qi, Y.-K.; Li, Z.; Du, S.-S. Bioorg. Chem. 2023, 134, 106451. |
| [80] | Kobayashi, K.; Taguchi, A.; Cui, Y.; Shida, H.; Muguruma, K.; Takayama, K.; Taniguchi, A.; Hayashi, Y. Eur. J. Org. Chem. 2021, 2021, 956. |
| [81] | Yin, H.; Fu, X.; Gao, H.; Gao, H.; Ma, Y.; Chen, X.; Zhang, X.; Du, S.-S.; Qi, Y.-K. Front. Oncol. 2023, 12, 1028600. |
| [82] | Qiao, Z.; Qi, H.; Zhang, H.; Zhou, Q.; Wei, N.; Zhang, Y.; Wang, K. Anal. Chem. 2020, 92, 1934. |
/
| 〈 |
|
〉 |