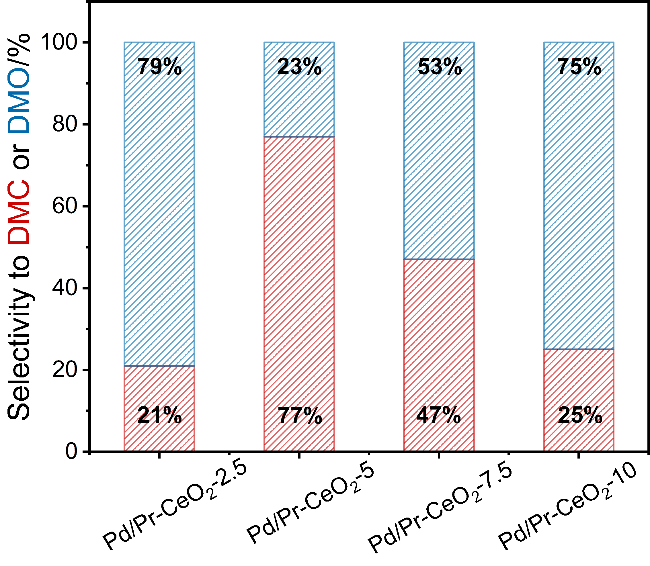
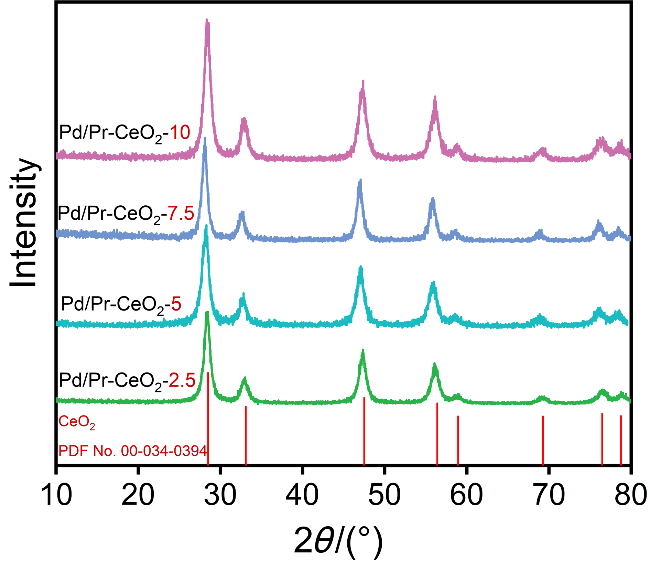
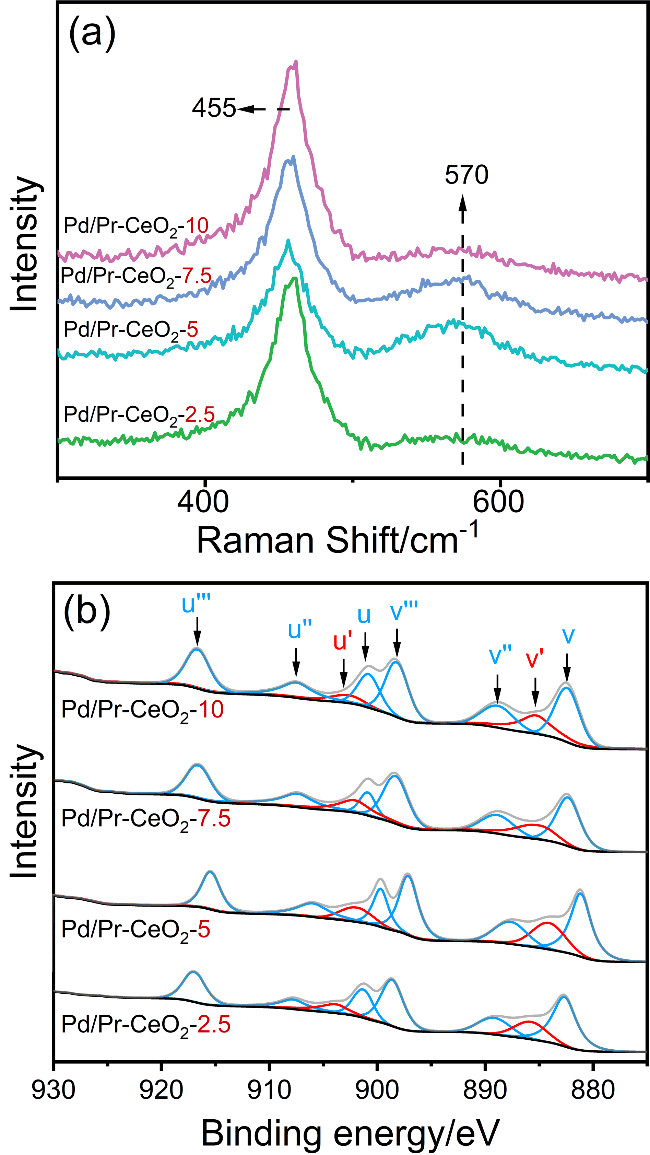
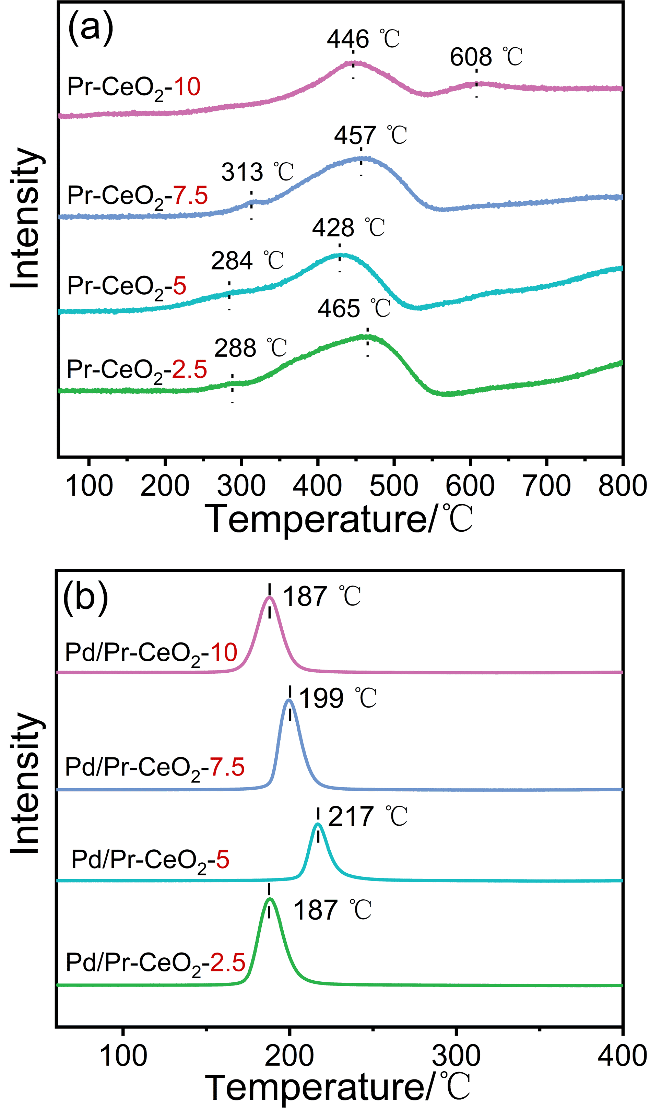
碳酸二甲酯(DMC)是一种绿色环保的精细化学品.由于DMC本身具有极性, 粘度低, 且较为稳定, 可作为优良有机溶剂
[1-2]. DMC可以代替甲基叔丁基醚作为绿色环保的汽油添加剂用于降低油品的凝固点, 调控其辛烷分布以及提升抗爆性
[3-4]. 由于具有较高的离子导电性, DMC在锂电池行业也被广泛用作电解液
[5-6]. DMC的生产技术包括光气法
[7]、酯交换法
[8]、尿素醇解法
[9-10]、CO
2直接合成法
[11]和CO酯化法. 其中CO酯化法在DMC合成过程中不产生水, 有利于稳定催化剂, 且反应条件温和, 具有独特的技术优势, 因此受到了广泛关注. 目前已经报道了一些含氯催化剂
[12-14]和无氯催化剂
[15-16], 尽管含氯催化剂表现出较高的初始催化活性和选择性, 但由于反应过程中氯离子会以氯甲酸甲酯副产物的形式不断流失, 导致催化剂迅速失活, 为了维持含氯催化剂的稳定性, 需要不断通入干燥的氯化氢气体, 造成了设备严重腐蚀, 增加了生产成本. 相比之下, 无氯催化剂可以摆脱氯离子的限制. 郭国聪等
[17]开发了一种孤立态的Pd(Ⅱ)/NaY无氯催化剂, 该催化剂能在100 h内保持对DMC的超高选择性(>99.5%). 报道的无氯催化剂的载体通常为NaY和UiO-66
[18-22]. 然而, NaY的酸性会造成原料亚硝酸甲酯(MN)的部分分解
[23], 产生不需要的副产物, 因此研发非分子筛体系的催化剂具有重要意义.