

Recent Advances of Photoinduced Deoxygenative Functionalization of Alcohol Derivatives
Received date: 2025-02-27
Online published: 2025-04-24
Supported by
National Natural Science Foundation of China(22161047)
National Natural Science Foundation of China(22301109)
Research Funds for Talent Introduction of Jiangsu Ocean University(KQ23065)
Lianyungang Haiyan Project(KK24005)
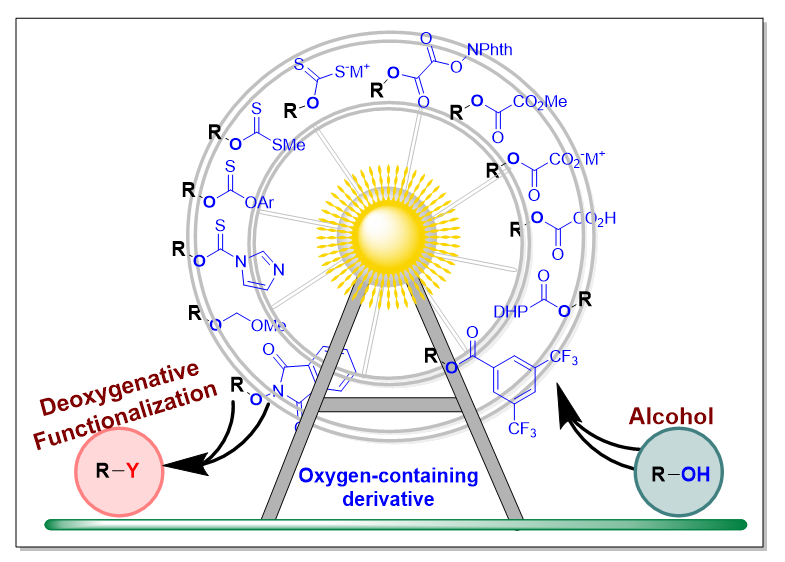
Alcohols are one of the most widespread existent in nature, and one of promising chemical feedstocks, due to their cheap and easy availability. Half a century ago, the Barton-McCombie deoxygenation was discovered, and continued to be improved by organic synthetic chemists, becoming an important and rather broad fields in modern organic chemistry. In the last decade, visible-light-driven photoredox catalysis is a powerful tool in organic synthesis, and can realize a good deal of chemical transformations, because of its milder reaction condition, functional group tolerance, high efficiency and environmental-friendly characteristics. It has been reported direct deoxygenation of alcohol in the previous literatures, including the photochemical reactions and metal-transition catalytic systems. Herein, we summarized and discussed some significant advances since in 2014, about the deoxygenative functionalization of alcohol derivatives in photochemical synthetic reactions. And oxygen-containing derivatives of alcohol mainly includes carboxylic esters, oxalic esters, thiocarbonates and their derivatives, ethers and others. In this minireview, in terms of their structural properties, we went to classify and introduce the catalytic modes and mechanisms of deoxygenation, reaction universalities, advantages and shortcomings. In these advances, the oxygen-containing derivatives of alcohol could proceed single electron oxidation and reduction processes, hydrogen atom transfer (HAT), as well as radical addition process, as to assist the C—O bond cleavage to yield the key alkyl radical intermediates, subsequent functionalization to construct the newly C—C, C—N, C—O, C—S, C—X (halo) and C—B bonds. In addition, we also outlooked the challenges and opportunities in the field of deoxygenation of alcohols, and the application prospects of some compounds containing the hydroxy group in the future was discussed as well.
Yu Zhao , Tongtong Xing , Yurong Duan , Quanqing Zhao . Recent Advances of Photoinduced Deoxygenative Functionalization of Alcohol Derivatives[J]. Acta Chimica Sinica, 2025 , 83(7) : 773 -802 . DOI: 10.6023/A25020057
| [1] |
|
| [2] |
|
| [3] |
(a)
(b)
|
| [4] |
(a)
(b)
(c)
|
| [5] |
(a)
(b)
(c)
(d)
(e) Ueng, S-H.;
(f)
(g)
|
| [6] |
|
| [7] |
Recent advances for photoredox catalysis: (a)
(b)
(c)
(d)
(e)
(f)
(g)
(h)
(i)
(j)
(k)
(l)
(李康葵, 龙先扬, 黄岳, 祝诗发, 化学学报, 2024, 82, 658.)
(m)
(归春明, 周潼瑶, 王海峰, 严琼姣, 汪伟, 黄锦, 陈芬儿, 有机化学, 2023, 43, 2647.)
|
| [8] |
(a)
(b)
(c)
(d)
(e)
(f)
(g)
|
| [9] |
Transition metal catalyzed the C—O bond activation: (a)
(b)
(c)
(d)
(e)
(f)
(g)
(h)
(i)
(j)
(k)
(l)
(m)
(n)
袁芳艳, 李超, 罗美明, 曾小明, 化学学报, 2023, 81, 456).
(o)
(p)
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
(a)
(b)
(c)
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
(a)
(b)
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
(a)
(b)
|
| [25] |
(a)
(b)
|
| [26] |
(a)
(b)
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
(a)
(b)
(c)
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
(a)
(b)
(c)
(d)
(e)
(f)
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
(a)
(b)
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
(a)
(b)
(c)
(d)
(e)
|
/
| 〈 |
|
〉 |