1 引言
2 结果与讨论
2.1 3D Eu-MOFs水凝胶的表征
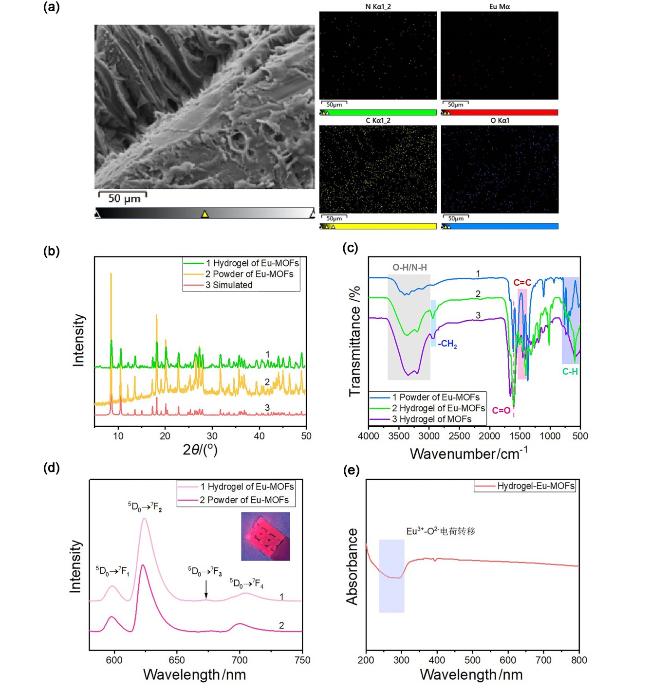
图1 (a) Eu-MOFs水凝胶的EDS图谱; (b) Eu-MOFs水凝胶和单晶Eu-MOFs粉体的XRD图谱; (c) Eu-MOFs水凝胶和单晶Eu-MOFs粉体的FT-IR; (d) Eu-MOFs水凝胶的稳态荧光发射光谱(PL), 插图为材料在365 nm紫外灯照射时的光学照片; (e) Eu-MOFs水凝胶的固体紫外-可见光谱图Figure 1 (a) EDS mapping of Eu-MOFs hydrogel; (b) XRD pattern of Eu-MOFs hydrogel and single crystal Eu-MOFs powder; (c) FT-IR spectra of Eu-MOFs hydrogel and single crystal Eu-MOFs powder; (d) Photoluminescence (PL) spectra of Eu-MOFs hydrogel, the insert is the optical photograph of Eu-MOFs hydrogel under the UV light with a wavelength of 365 nm; (e) UV-vis spectra of Eu-MOFs hydrogel |
2.2 3D Eu-MOFs水凝胶对丙酮的选择性识别
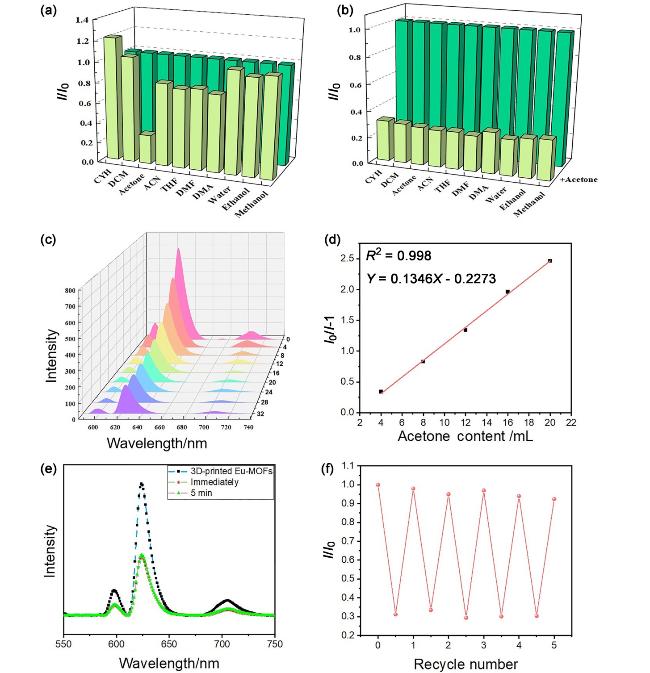
图2 (a) Eu-MOFs水凝胶在不同溶剂添加前(深绿色)和添加后(浅黄色)的荧光强度对比; (b) Eu-MOFs水凝胶在体积分数5%其他溶剂与体积分数95%丙酮混合前和混合后(浅黄色)的荧光强度对比; (c) Eu-MOFs水凝胶荧光强度随丙酮体积变化的光谱图(体积单位: μL); (d)通过Stern-Volmer方程得到添加丙酮的体积与水凝胶荧光强度变化相对值的线性关系; (e)添加丙酮后, Eu-MOFs水凝胶荧光强度的时间响应; (f) 5个循环过程中, Eu-MOFs水凝胶荧光强度的变化, 其中I0为初始荧光强度, I为循环过程中荧光强度检测值Figure 2 (a) Comparison of fluorescence intensity of Eu-MOFs hydrogel before (dark green) and after (light yellow) addition of different solvents; (b) Comparison of fluorescence intensity of Eu-MOFs hydrogel before (dark green) and after (light yellow) mixing 5% (volume fraction) other solvents with 95% (volume fraction) acetone; (c) Spectrogram of fluorescence intensity of Eu-MOFs hydrogel as a function of acetone volume (volume unit: μL); (d) The linear relationship between the volume of the added acetone and the relative value of the fluorescence intensity change of the hydrogel was obtained by the Stern-Volmer equation; (e) Time response of fluorescence intensity of Eu-MOFs hydrogel after acetone addition; (f) The change of fluorescence intensity of Eu-MOFs hydrogel during five cycles, where I0 is the initial fluorescence intensity, and I is the detection value of fluorescence intensity during the cycle. |
表1 不同MOFs传感器对丙酮的传感性能比较Table 1 Comparison of acetone sensing performance of different sensors of MOFs |
| MOF | 灵敏度/检出限 | 可逆性 | Ref. |
|---|---|---|---|
| [Eu5(DBA)3]n | LOD: 1.24 μmol/L | — | [40] |
| [Me2NH2]2[(Eu)2(ofdp)2(DMF)(H2O)]•7H2O•DMF | LOD: 7.4% (volume fraction) | — | [41] |
| MOF-808-Tb | 浓度低于8600 μmol/L, 荧光强度降低到近50% | 是 | [42] |
| [Cd(dcba)(DMA)]•DMA | Ksv: 8.4×104 L•mol-1 | 是 | [43] |
| DhaTab-COF-EuIL | LOD: 1.0% (volume fraction) | — | [44] |
| Eu/Tb@Bi-MOF | — | — | [45] |
| Eu-BPDA | 丙酮浓度0.028% (volume fraction)时, 荧光强度降低50% | 是 | [46] |
| 3D-printed Eu-MOFs | LOD: 2.43 μL | 是 | 本工作 |
2.3 3D Eu-MOFs水凝胶对丙酮传感机制的探究
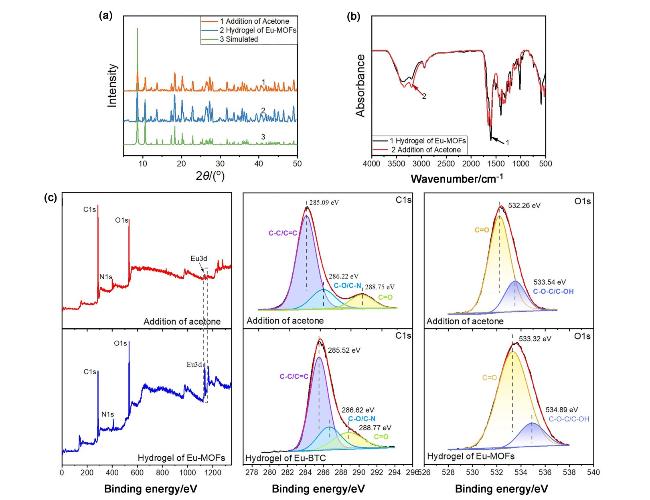
图3 (a) Eu-MOFs水凝胶添加丙酮前后的X射线衍射图谱; (b) Eu-MOFs水凝胶添加丙酮前后的红外图谱; (c)丙酮添加前后Eu-MOFs水凝胶的XPS光谱和精细谱.Figure 3 (a) X-ray diffraction patterns of Eu MOFs hydrogel before and after adding acetone; (b) FT-IR spectra of Eu MOFs hydrogel before and after adding acetone; (c) XPS survey spectra and high-resolution spectra of Eu MOFs hydrogel before and after acetone addition |
2.4 3D Eu-MOFs水凝胶对PNA的选择性识别
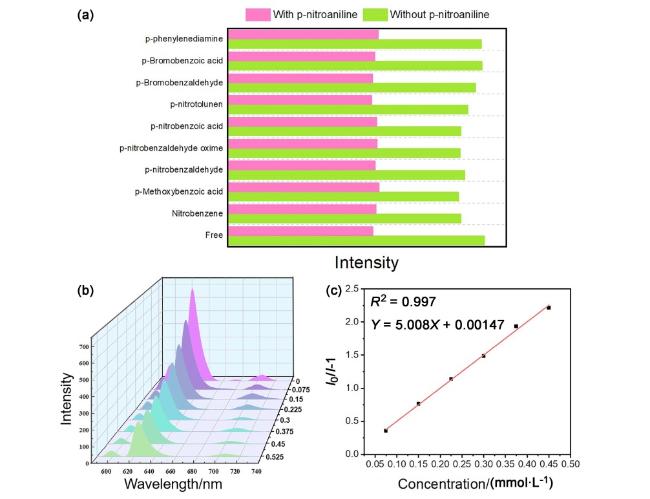
图4 (a) Eu-MOFs水凝胶对不同有机小分子的荧光响应, 每个有机小分子的浓度为0.15 mmol•L-1. 绿色条表示不存在PNA时的荧光强度, 红色条表示存在PNA时的荧光强度; (b)不同PNA浓度下, Eu-MOFs水凝胶的荧光强度(浓度单位: mmol•L-1); (c)通过Stern-Volmer方程得到不同浓度的PNA与水凝胶荧光强度变化相对值的线性关系Figure 4 (a) Fluorescence response of Eu-MOFs hydrogel to different organic small molecules, and the concentration of each organic small molecule is 0.15 mmol•L-1. The green bar represents the fluorescence intensity in the absence of PNA, while the red bar represents the fluorescence intensity in the presence of PNA; (b) spectrogram of fluorescence intensity of Eu-MOFs hydrogel changing with PNA concentration (concentration unit: mmol•L-1); (c) the linear relationship between PNA of different concentrations and the relative value of fluorescence intensity of hydrogel was obtained by Stern-Volmer equation |
2.5 3D Eu-MOFs水凝胶对PNA识别的机理探究
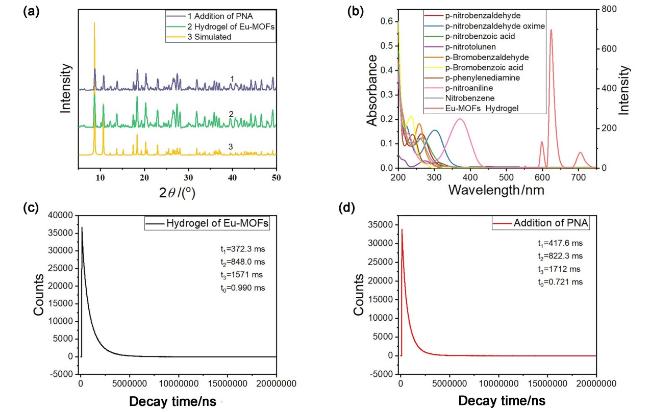
图5 (a) Eu-MOFs水凝胶添加丙酮前后的X射线衍射图谱; (b)有机小分子的吸收光谱与Eu-MOFs水凝胶的发射光谱; (c)不含有PNA和(d)含有PNA的Eu-MOFs水凝胶的时间衰减曲线Figure 5 (a) X-ray diffraction patterns of Eu MOFs hydrogel before and after adding acetone; (b) absorption spectra of small organic molecules and emission spectra of Eu-MOF hydrogels; (c) time-resolved decay curve of Eu MOFs hydrogels with PNA and (d) without PNA |