

Total Synthesis of Complex Natural Products Via Radical-Mediated Formal Diels-Alder Reaction
Received date: 2025-04-24
Online published: 2025-05-23
Supported by
National Natural Science Foundation of China(22371117)
Science and Technology Innovation Program of Hunan Province(2022RC1106)
In recent years, the radical-mediated formal Diels-Alder reaction of dienophiles and dienes under mild conditions has provided a novel approach for the stereoselective construction of six-membered ring skeleton. This review offers a concise overview of the application of radical-mediated formal Diels-Alder reactions in the total synthesis of bioactive complex natural products. It mainly includes three types of reaction pathways: (1) the radical cation-mediated formal Diels-Alder reaction, (2) the single-electron-transfer-initiated formal Diels-Alder reaction, and the diradical-involved formal Diels-Alder reaction, which enable the stereoselective generation of target ring systems.
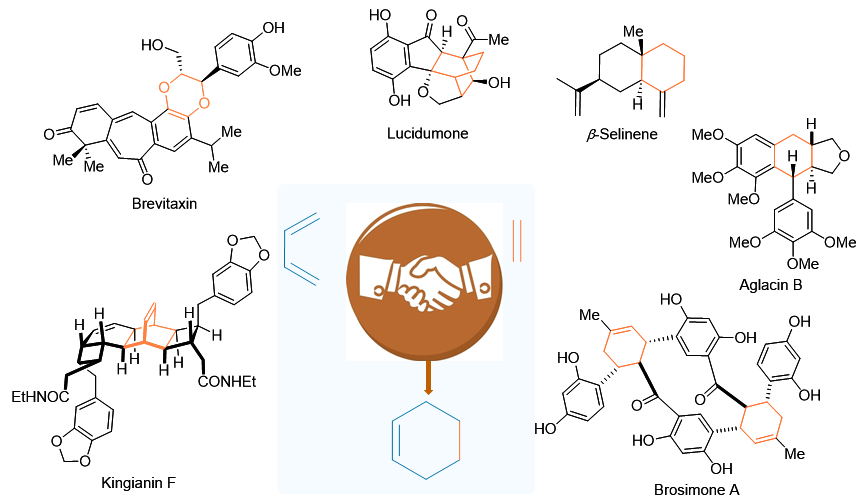
Huang Jun , Yin Rong , Cao Tingting . Total Synthesis of Complex Natural Products Via Radical-Mediated Formal Diels-Alder Reaction[J]. Acta Chimica Sinica, 2025 , 83(11) : 1424 -1434 . DOI: 10.6023/A25040131
| [1] |
(a)
(b)
|
| [2] |
(a)
(b)
(c)
(d)
(e)
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
(a)
(b)
(c)
(d)
|
| [8] |
(a)
(b)
(c)
(d)
(吕健, 钟兴仁, 程津培, 罗三中, 化学学报, 2012, 70, 1518.)
(e)
(f)
(g)
(周远春, 周志, 杜玮, 陈应春, 化学学报, 2018, 76, 382.)
(h)
(i)
(j)
(k)
(l)
(m)
(n)
(o)
(丁祥峰, 邓卫平, 有机化学, 2020, 40, 3976.)
|
| [9] |
(a)
(b)
(c)
(d)
(e)
(f)
(g)
(h)
(i)
(j)
(k)
(l)
(m)
(n)
(o)
(p)
(q)
(r)
|
| [10] |
(a)
(b)
(c)
(d)
(熊兴泉, 陈会新, 有机化学, 2013, 33, 1437.)
|
| [11] |
(a)
(b)
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
(a)
(b)
(c)
|
| [18] |
|
| [19] |
(a)
(b)
(c)
|
| [20] |
(a)
(b)
|
| [21] |
(a)
(b)
|
| [22] |
(a)
(b)
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
(a)
(b)
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
(a)
(b)
|
| [41] |
(a)
(b)
(c)
(d)
|
| [42] |
|
| [43] |
(a)
(b)
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
(a)
(b)
(c)
(d)
(e)
(f)
|
| [53] |
|
| [54] |
(a)
(b)
(c)
|
| [55] |
(a)
(b)
(c)
|
| [56] |
|
| [57] |
|
| [58] |
(a)
(b)
(c)
(d)
(e)
(f)
|
| [59] |
(a)
(b)
|
| [60] |
|
| [61] |
(a)
(b)
|
| [62] |
(a)
(b)
(c)
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
(a)
(b)
|
| [75] |
|
| [76] |
|
| [77] |
(a)
(b)
|
| [78] |
|
| [79] |
(a)
(b)
(c)
|
| [80] |
|
| [81] |
|
| [82] |
(a)
(b)
(c)
|
| [83] |
(a)
(b)
(c)
|
/
| 〈 |
|
〉 |