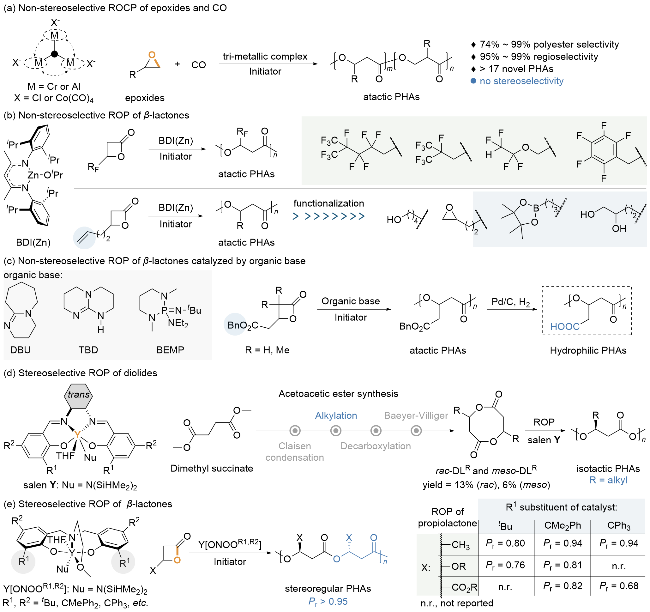
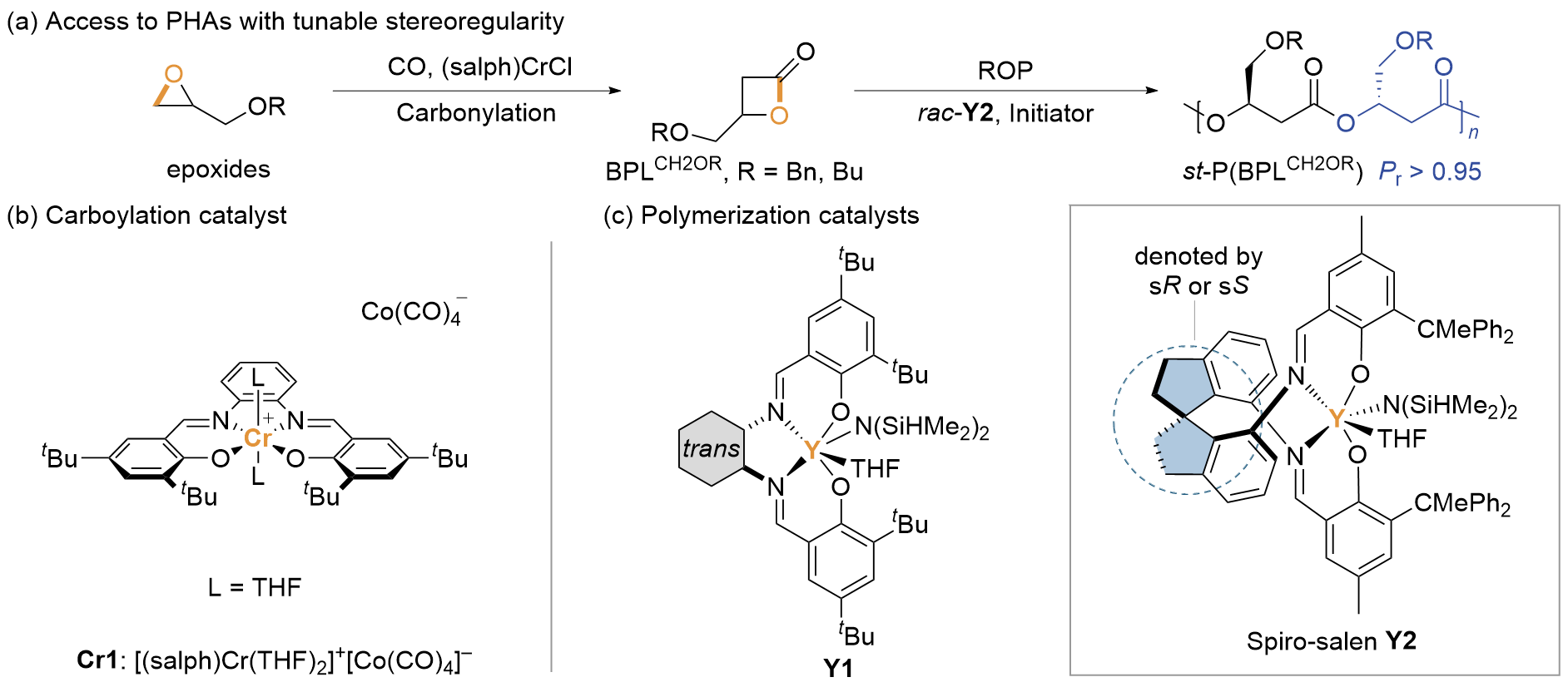
1 引言
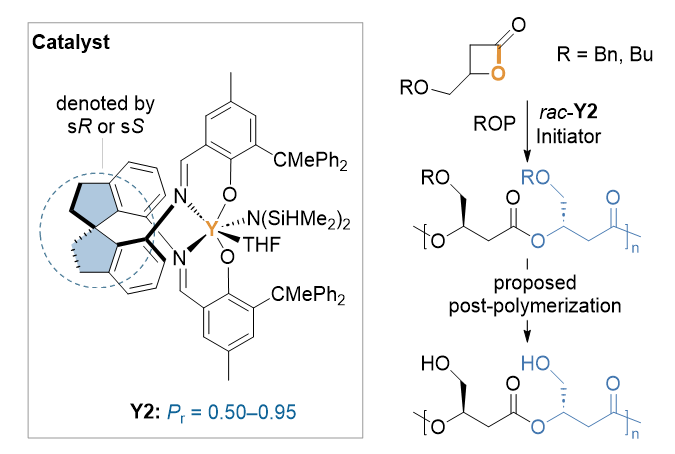
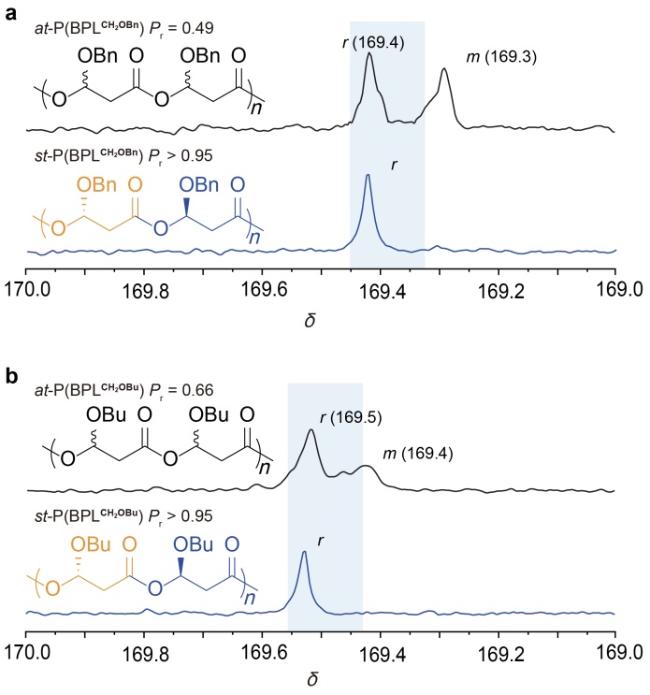
表1 螺环-salen配合物催化的取代丙内酯立体选择性开环聚合aTable 1 Stereoselective ROP of substituted propiolactones mediated by spiro-salen complexes |
| Entry | Monomer | Catalyst | Timeb | Conv.c/% | Mnd/kDa | Đd (Mw/Mn) | Pre |
|---|---|---|---|---|---|---|---|
| 1 | rac-BPLCH2OBu | rac-Y1 | 10 min 12 h | 17 51 | n.d.f 1.24 | n.d.f 1.27 | n.d.f 0.50 |
| 2 | rac-BPLCH2OBu | (R)-Y2 | 3.5 h | 73 | 7.59 | 1.07 | 0.66 |
| 3 | rac-BPLCH2OBu | rac-Y2 | 1 h | >99 | 8.26 | 1.12 | >0.95 |
| 4 | rac-BPLCH2OBn | rac-Y1 | 10 min 12 h | n.d.f 85 | n.d.f 1.06 | n.d.f 1.05 | n.d.f 0.65 |
| 5 | rac-BPLCH2OBn | (R)-Y1 | 6 h | 30 | n.d.f | n.d.f | 0.60 |
| 6 | rac-BPLCH2OBn | (R)-Y2 | 3.5 h | 79 | 8.50 | 1.15 | 0.49 |
| 7 | rac-BPLCH2OBn | rac-Y2 | 20 s | >99 | 10.7 | 1.13 | >0.95 |
a Conditions: monomer=1 mmol, [Monomer]=2.0 mol/L, p-tolylmethanol (PTMA) as the initiator, Toluene as the solvent, room temperature, [Monomer]/[Catalyst]/[Initiator]=200/1/1. b The reaction time was not optimized. c Monomer conversion measured by 1H NMR of the quenched solution. d Number-average molecular weight (Mn) and dispersity index (Đ=Mw/Mn), determined by size exclusion chromatography (SEC) at 40 ℃ in THF. e Pr is the probability of racemic linkages between monomer units determined by 13C NMR spectroscopy. f Not detected. |