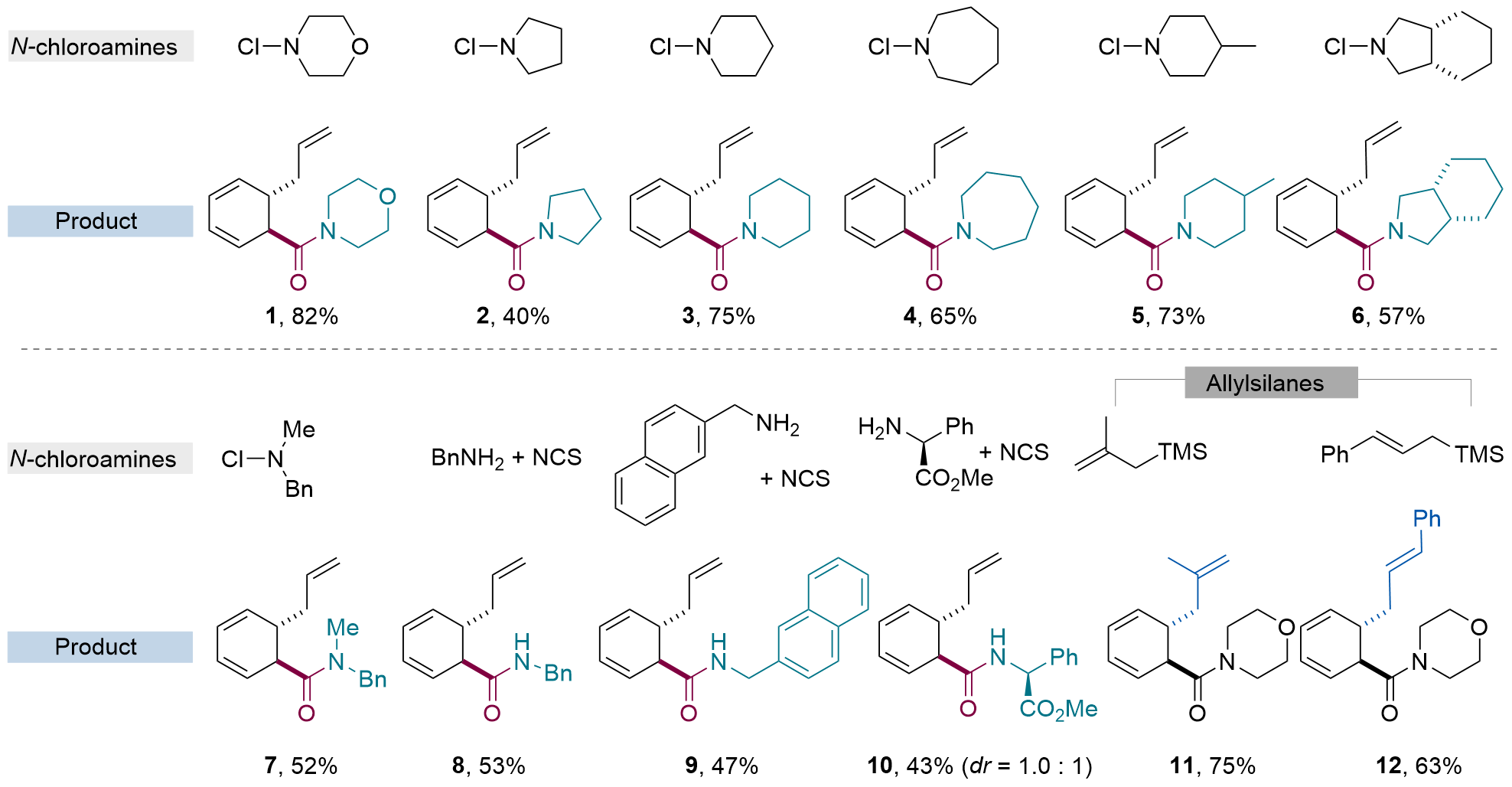
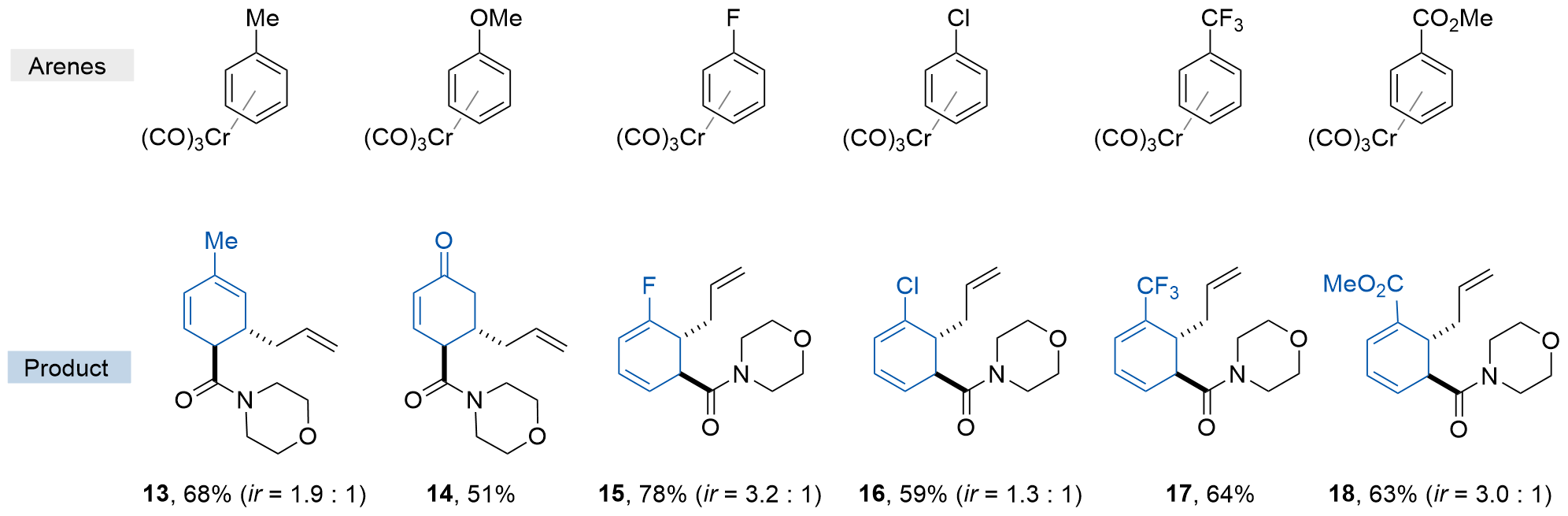
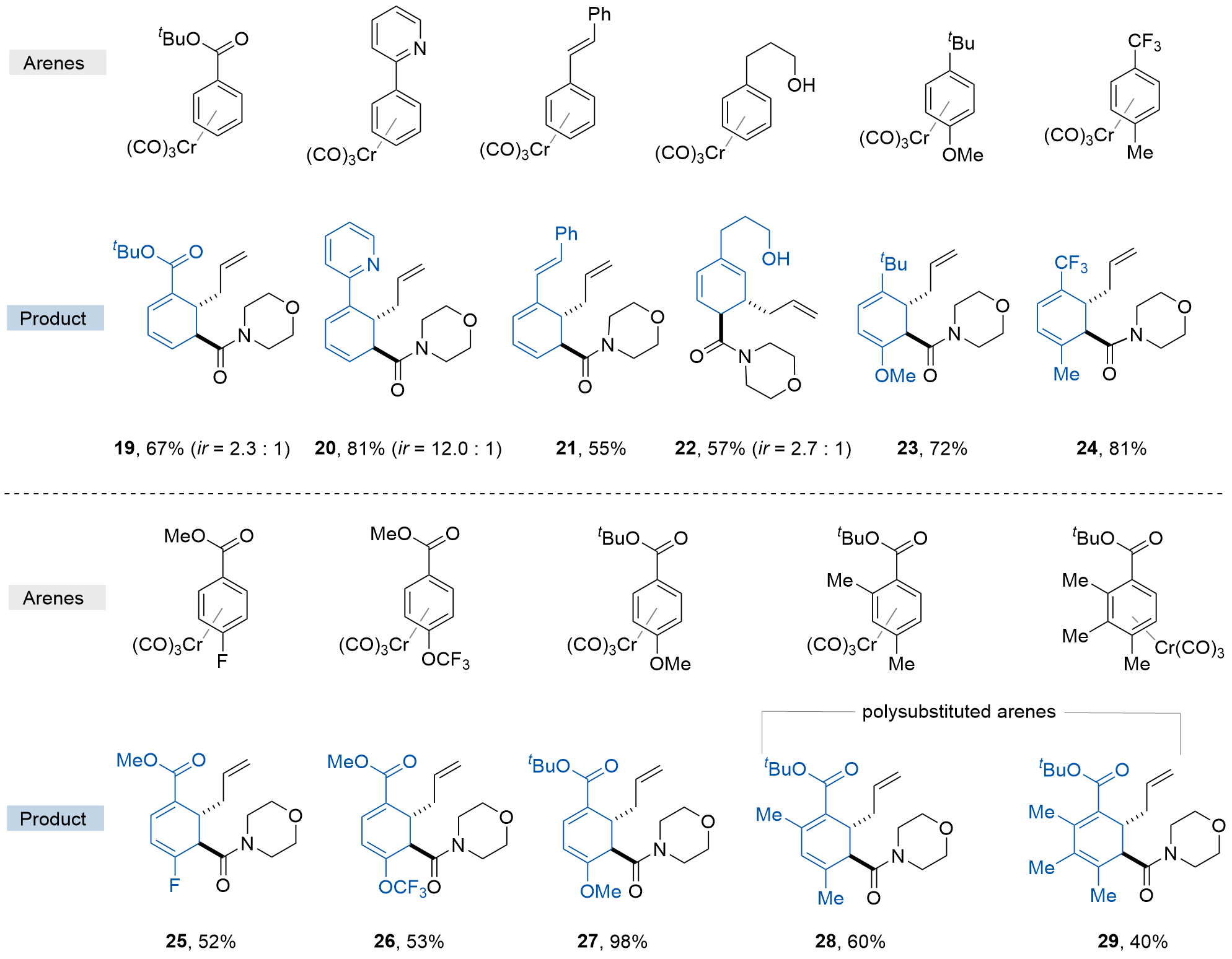
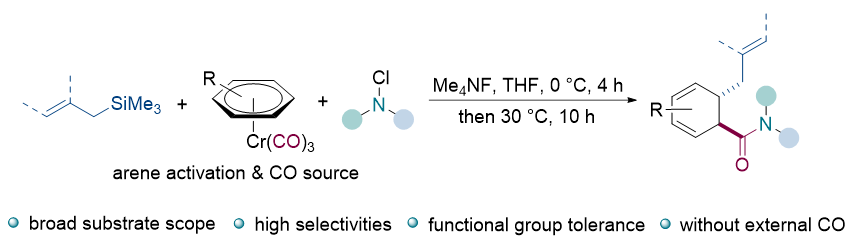
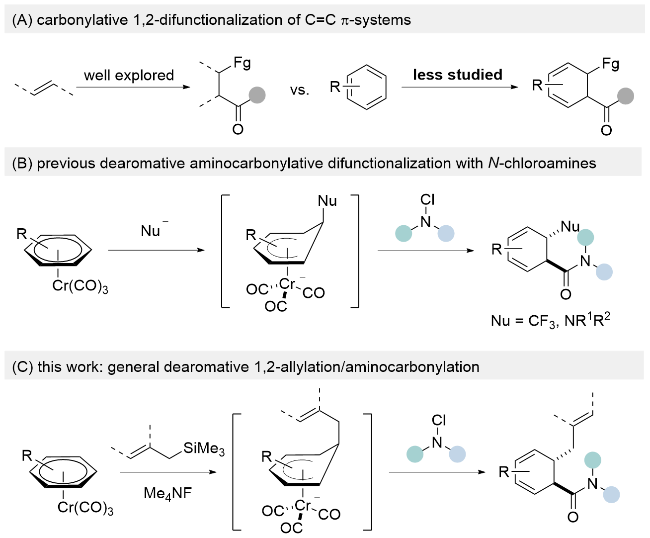
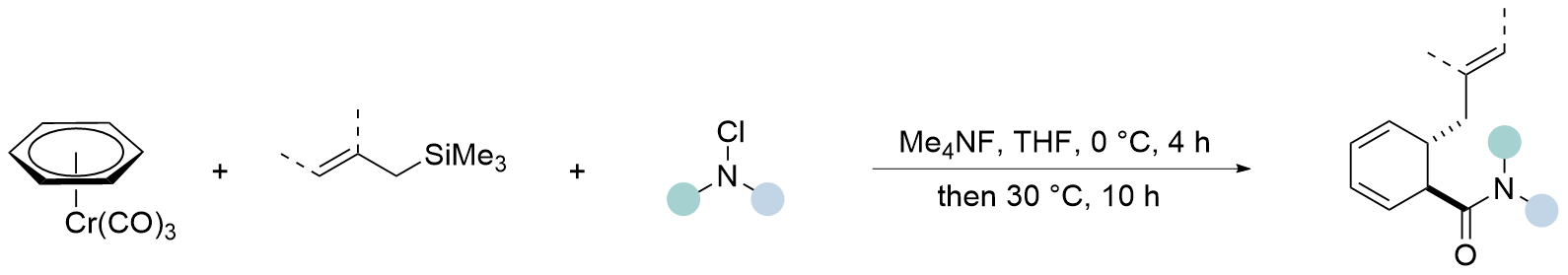
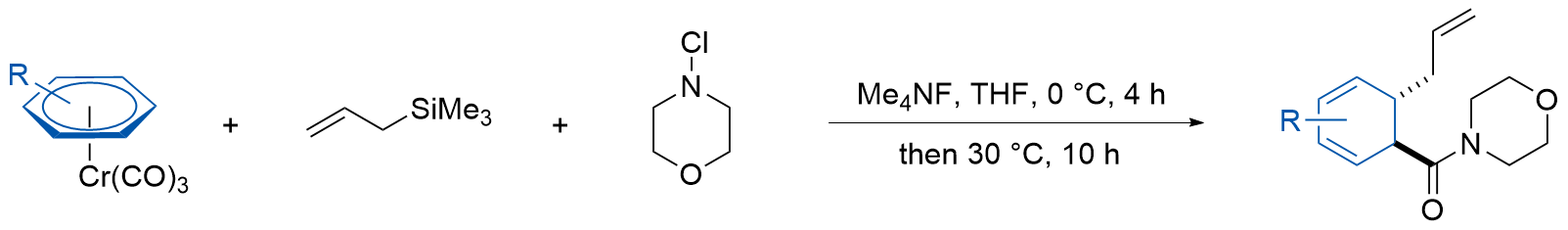
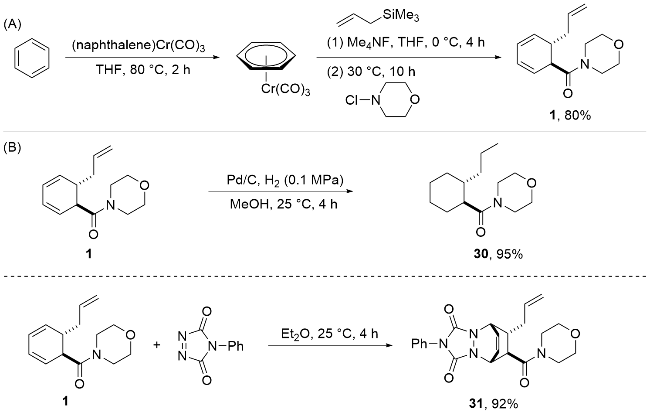
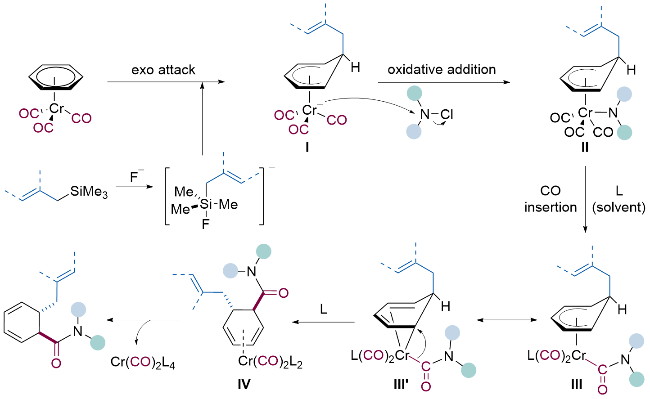
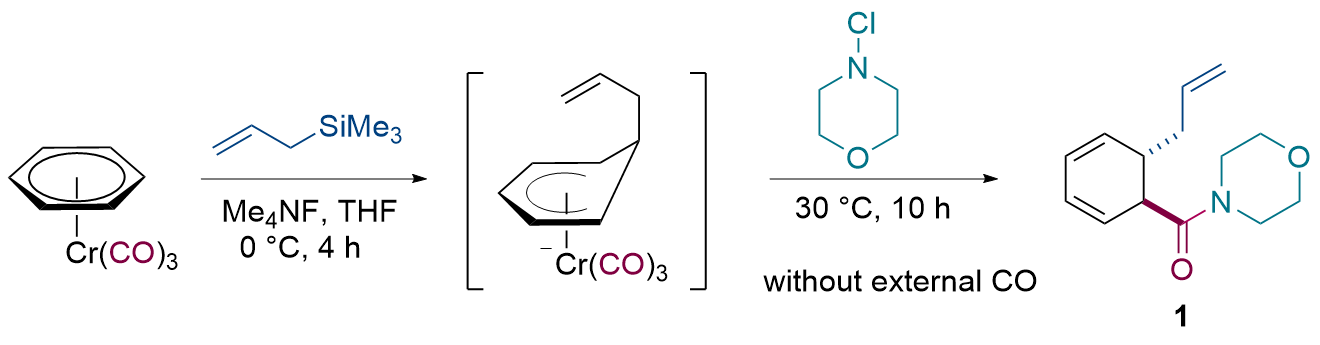| [12] |
(a) Greenberg, A.; Breneman, C. M.; Liebman, J. F. The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science, Wiley, New York, 2000.
(b) Allen, C. L.; Williams, J. M. J. Chem. Soc. Rev. 2011, 40, 3405.
(c) Guo, X.; Facchetti, A.; Marks, T.-J. Chem. Rev. 2014, 114, 8943.
(d) Brown, D. G.; Boström, J. J. Med. Chem. 2016, 59, 4443.
DOI
PMID
(e) Kumari, S.; Carmona, A. V.; Tiwari, A. K.; Trippier, P. C. J. Med. Chem. 2020, 63, 12290.
(f) Li, L.; Zhou, B.; Ye, L. Chin. J. Org. Chem. 2015, 35, 655 (in Chinese).
(李龙, 周波, 叶龙武, 有机化学, 2015, 35, 655.)
DOI
(g) Li, Y.; Jia, F.; Ma, L.-N.; Li, Z.-P. Acta Chim. Sinica 2015, 73, 1311 (in Chinese).
(李远明, 贾凡, 马丽娜, 李志平, 化学学报, 2015, 73, 1311.)
DOI
(h) Huang, P.-Q. Acta Chim. Sinica, 2018, 76, 357 (in Chinese).
(黄培强, 化学学报, 2018, 76, 357.)
DOI
(i) Li, C.; Wang, M.; Lu, X.; Yang, Y.; Zhang, L. Chin. J. Org. Chem. 2019, 39, 1109 (in Chinese).
(李春, 王梦娜, 陆逊花, 杨元勇, 张林, 有机化学, 2019, 39, 1109.)
DOI
(j) Li, G.-K.; Zhu, B.-F.; Hu, T.; Fan, R.-F.; Sun, W.-Q.; He, Z.-X.; Chen, J.-C.; Fan, B.-M. Acta Chim. Sinica 2025, 83, 199 (in Chinese).
(李国凯, 朱滨锋, 胡涛, 樊瑞峰, 孙蔚青, 和振秀, 陈景超, 樊保敏, 化学学报, 2025, 83, 199.)
DOI
|
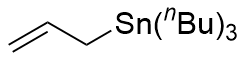 instead of
instead of