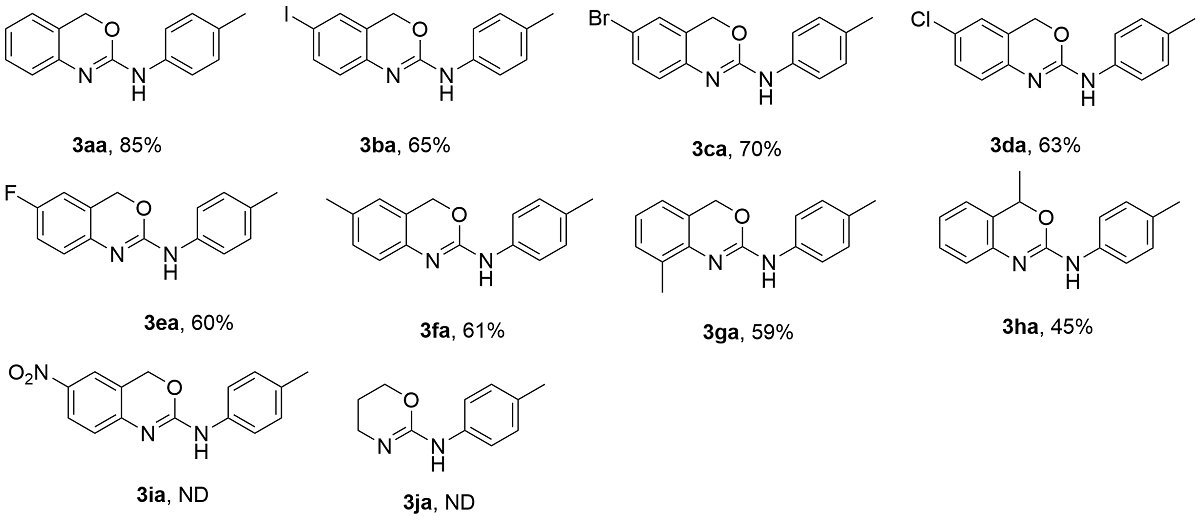
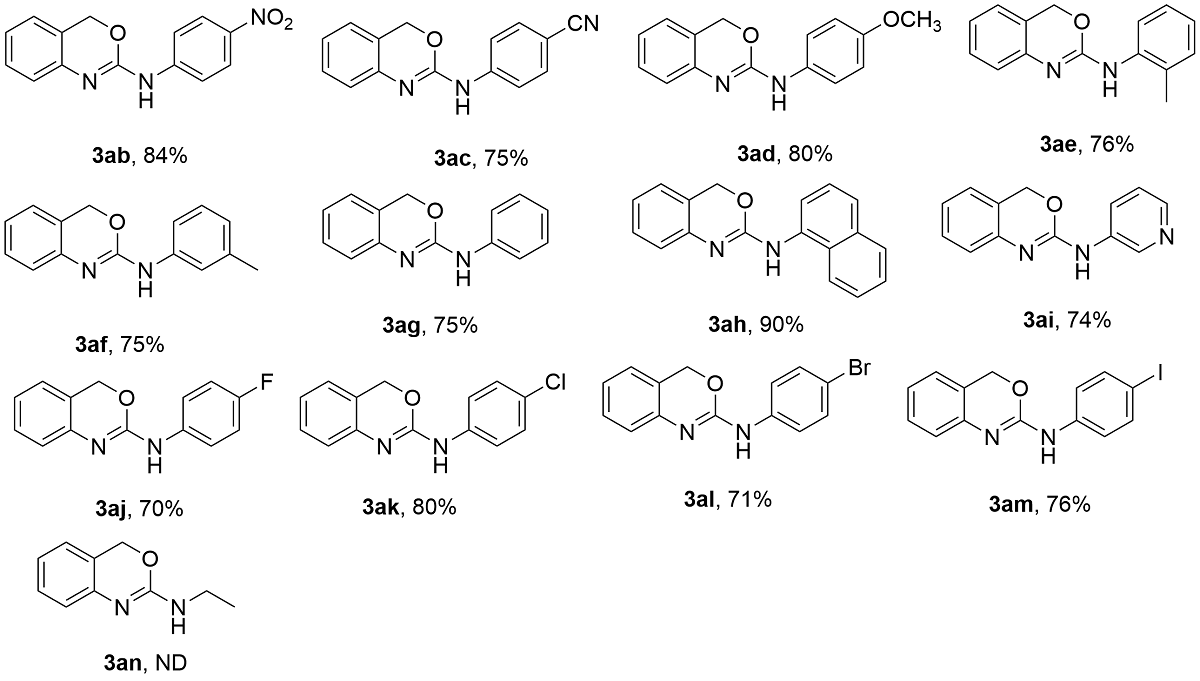
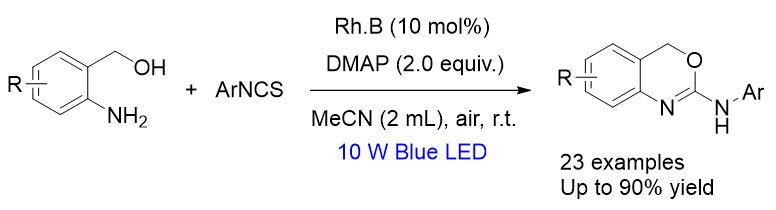
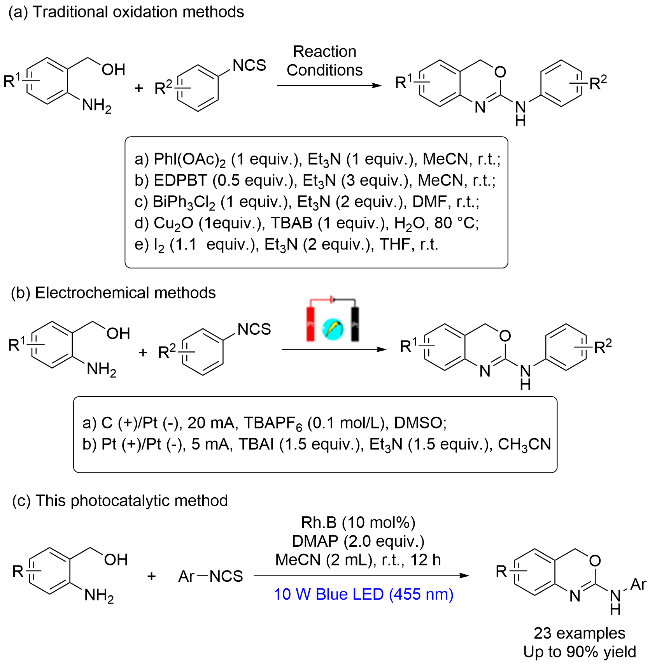
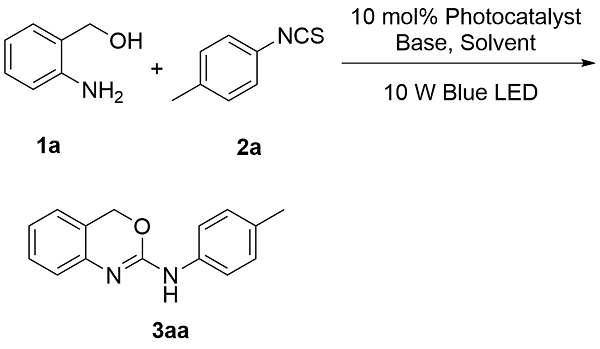
为探究2-氨基-1,3-苯并噁嗪(
3aa)的光催化合成最佳反应条件, 本研究以2-氨基苯甲醇(
1a)与对甲苯异硫氰酸酯(
2a)为模板底物进行条件筛选(
表1). 初始反应体系采用10 mol%的罗丹明B (Rh.B)为光催化剂、4-二甲氨基吡啶(DMAP)为碱、
N,
N-二甲基甲酰胺(DMF)为溶剂, 在室温条件下经10 W蓝色LED光源照射12 h, 目标产物
3aa的产率为41%(
表1, Entry 1). 通过溶剂筛选实验(
表1, Entries 2~7), 依次考察了二甲基亚砜(DMSO)、1,2-二氯乙烷(DCE)、乙醇(EtOH)、乙腈(MeCN)、甲苯(Toluene)和1,4-二氧六环(1,4-dioxane)的替代效果, 结果显示乙腈(MeCN)为最佳溶剂, 产率提升至85%. 随后系统评估不同碱的影响(
表1, Entries 8~14), 包括1,8-二氮杂二环十一碳-7-烯(DBU)、氢氧化钾(KOH)、叔丁醇钾(
t-BuOK)、1,5-二氮杂二环[4.3.0]壬-5-烯(DBN)、碳酸钾(K
2CO
3)、三乙胺(Et
3N)和磷酸三钠(Na
3PO
4), 最终确认4-二甲氨基吡啶(DMAP)仍为最优碱. 进一步筛选光催化剂(
表1, Entries 15~18), 对比曙红Y (Eosin Y)、玫瑰红(Rose Bengal)、三(2,2'-联吡啶)二氯化钌([Ru(bpy)
3]Cl
2)和亚甲基蓝(Methylene Blue)的效果, 发现罗丹明B的综合性能最佳. 控制实验表明: 光照是该体系的关键因素, 无光照时产率低于5%; 未添加Rh.B时产率降至10%, 证实其光催化必要性(
表1, Entries 19~20).