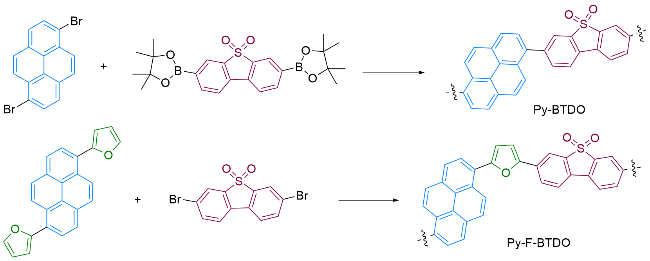
1 引言
2 结果与讨论
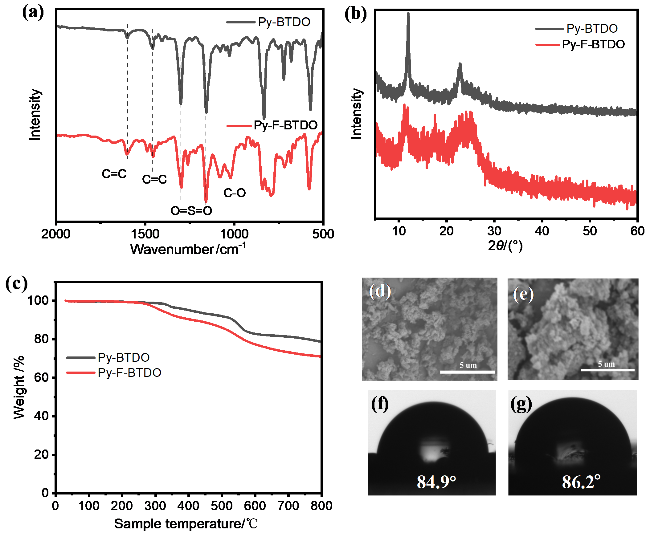
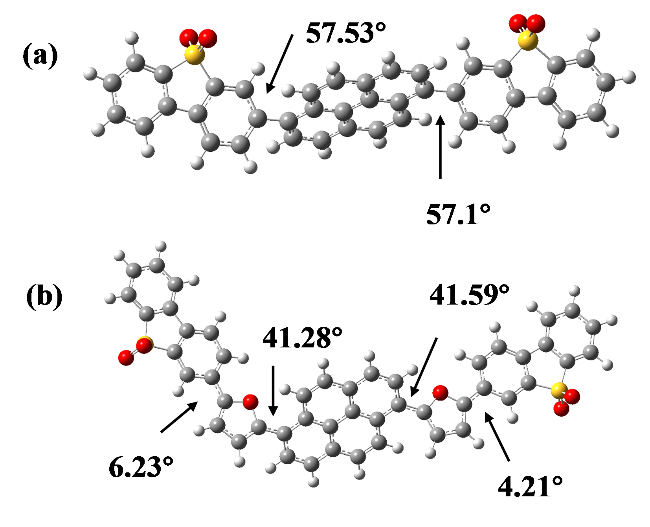
2.1 聚合物的结构表征与物理性能
图2 聚合物的(a)红外波谱图, (b)粉末XRD图, (c)热失重分析曲线, Py-BTDO (d)和Py-F-BTDO (e)的扫描电子显微镜照片, Py-BTDO(f)和Py-F-BTDO(g)的接触角照片Figure 2 (a) FT-IR spectra of the polymer, (b) powder XRD pattern, (c) thermogravimetric analysis curve, scanning electron microscopy images of Py BTDO (d) and Py-F-BTDO (e), contact angle images of Py BTDO (f) and Py-F-BTDO (g) |
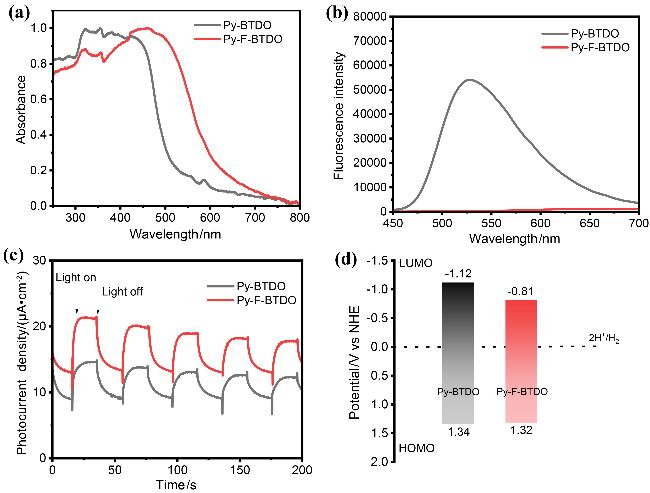
2.2 聚合物的光物理与电化学性能
2.3 聚合物的理论计算和轨道模拟
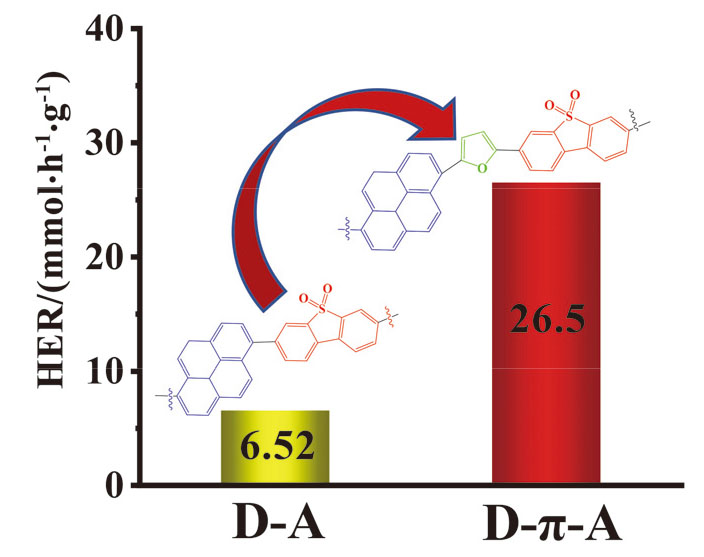
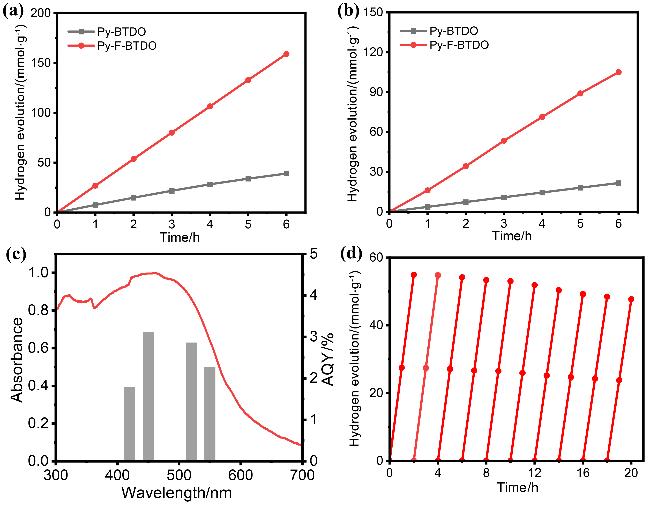
2.4 聚合物的光催化产氢性能
图5 聚合物在(a) λ>300 nm和(b) λ>420 nm下的光催化产氢活性, (c) Py-F-BTDO的紫外可见吸收光谱图和AQY, (d) Py-F-BTDO在全弧光(λ>300 nm)下的稳定性测试Figure 5 The photocatalytic hydrogen evolution activity of polymer under (a) ultraviolet visible light (λ>300 nm) and (b) visible light (λ>420 nm), (c) UV visible absorption spectra of Py-F-BTDO and stability testing of AQY, (d) Py-F-BTDO under full arc light (λ>300 nm) |
表1 聚合物的带隙和制氢速率Table 1 Band gap and hydrogen production rate of polymers |
| Polymer | Ega/eV | HERb/ (mmol•h-1•g-1) | HERc/ (mmol•h-1•g-1) |
|---|---|---|---|
| Py-BTDO | 2.46 | 6.52 | 3.6 |
| Py-F-BTDO | 2.13 | 26.5 | 17.5 |
a Band gap; b photocatalytic hydrogen evolution rate under UV visible light (λ>300 nm); c photocatalytic hydrogen evolution rate under visible light (λ>420 nm). |
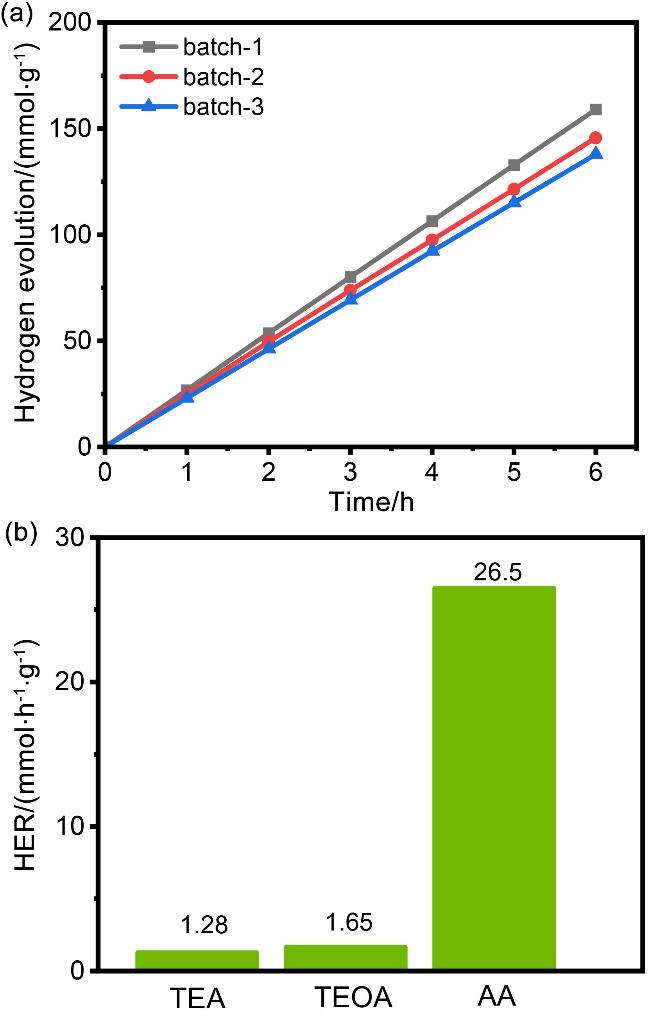
2.5 聚合物光催化产氢的重复性
图6 (a)不同批次Py-F-BTDO在全弧光照射下(λ>300 nm)的析氢活性; (b)紫外-可见光照射下不同牺牲剂的光催化产氢速率Figure 6 (a) The hydrogen evolution activity of Py-F-BTDO produced from different batches under full arc light irradiation (λ>300 nm); (b) photocatalytic hydrogen production rate of different sacrificial agents under UV visible light irradiation |