1 引言
2 结果与讨论
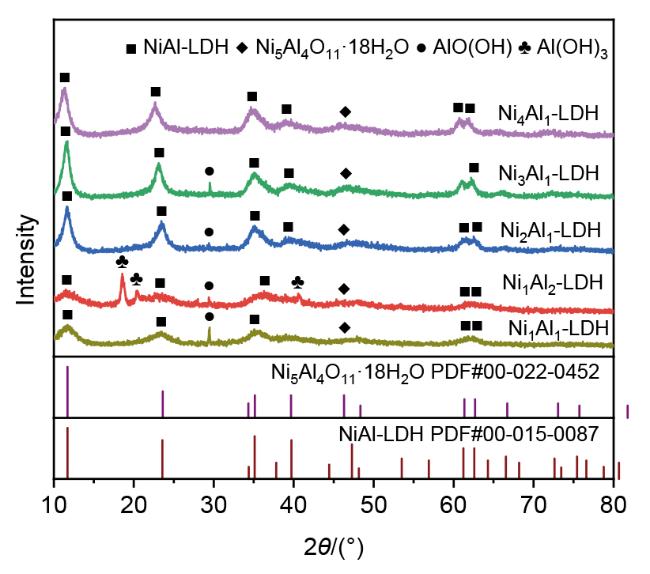
2.1 催化剂表征
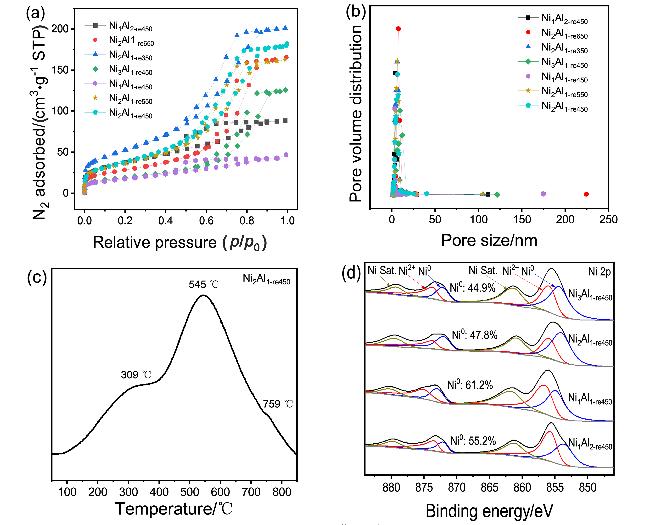
图3 (a) N2吸附-脱附等温线; (b)所有样品的孔径分布; (c) Ni2Al1-re450的CO-TPD图谱; (d) Ni1Al2-re450, Ni1Al1-re450, Ni2Al1-re450和Ni3Al1-re450的XPS图谱Figure 3 (a) N2 adsorption-desorption isotherm; (b) pore size distribution of all samples; (c) CO-TPD spectra of Ni2Al1-re450; (d) XPS Spectra of Ni1Al2-re450, Ni1Al1-re450, Ni2Al1-re450 and Ni3Al1-re450 |
表1 制备的催化剂样品的规格Table 1 Specifications of the prepared catalyst samples |
| Entry | Catalyst | SBETa/(m2•g-1) | Vporeb/(cm3•g-1) | Dporec/nm | wNid/% | Dse |
|---|---|---|---|---|---|---|
| 1 | Ni1Al2-re450 | 135 | 0.14 | 3.8 | — | — |
| 2 | Ni1Al1-re450 | 64 | 0.07 | 3.8 | — | — |
| 3 | Ni2Al1-re450 | 142 | 0.28 | 5.3 | 53.28 | 0.5568 |
| 4 | Ni3Al1-re450 | 68 | 0.21 | 9.6 | — | — |
| 5 | Ni2Al1-re350 | 189 | 0.32 | 4.3 | — | — |
| 6 | Ni2Al1-re550 | 130 | 0.27 | 6.5 | — | — |
| 7 | Ni2Al1-re650 | 101 | 0.28 | 7.8 | — | — |
a Specific surface area. b Mean pore radius. c Pore volume. d Ni contents analyzed by ICP-OES. e The dispersion of nickel metal was calculated by CO-TPD. |
2.2 间歇式反应釜中氮甲基吲哚加氢研究
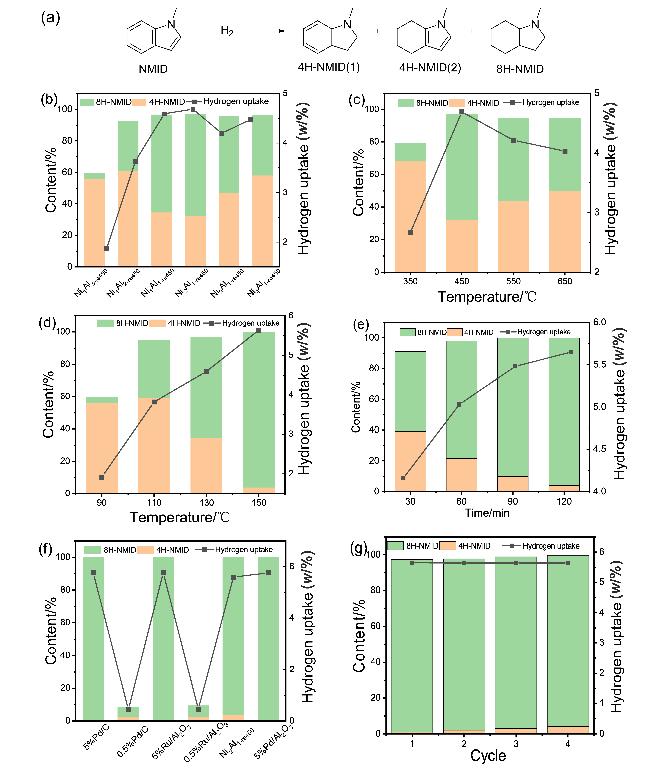
图4 (a) NMID氢化产物; (b)不同Ni/Al物质的量比; (c)不同还原温度; (d)不同反应温度; (e)反应时间对Ni2Al1-re450催化加氢NMID的影响; (f)与贵金属催化剂对比; (g)循环实验Figure 4 (a) NMID hydrogenation products; (b) effect of different Ni/Al molar ratios; (c) effect of different reduction temperatures; (d) effect of different reaction temperatures; (e) effect of reaction time on Ni2Al1-re450 hydrogenation NMID; (f) comparison with noble metal catalysts; (g) cycle experiment; (reaction conditions: 0.2 g NMID, 20 mg catalyst) |
图5 (a) ln(1-x)和反应时间之间的关系; (b) ln k和(1/T)之间的关系; (c)热过滤实验; (d), (e), (f) NMID在Ni2Al1-re450催化剂上的产物分布(100 ℃, 110 ℃, 130 ℃, 6 MPa H2). 反应条件: 0.2 g NMID, 20 mg催化剂Figure 5 (a) Relationship between ln(1-x) and reaction time; (b) relationship between ln k and 1/T; (c) thermal filtration experiment; (d), (e), (f) product distribution over Ni2Al1-re450 catalyst at various temperatures (100 ℃, 110 ℃, 130 ℃, 6 MPa H2). Reaction conditions: 0.2 g NMID, 20 mg catalyst |
2.3 微填充床反应器中氮甲基吲哚连续加氢研究
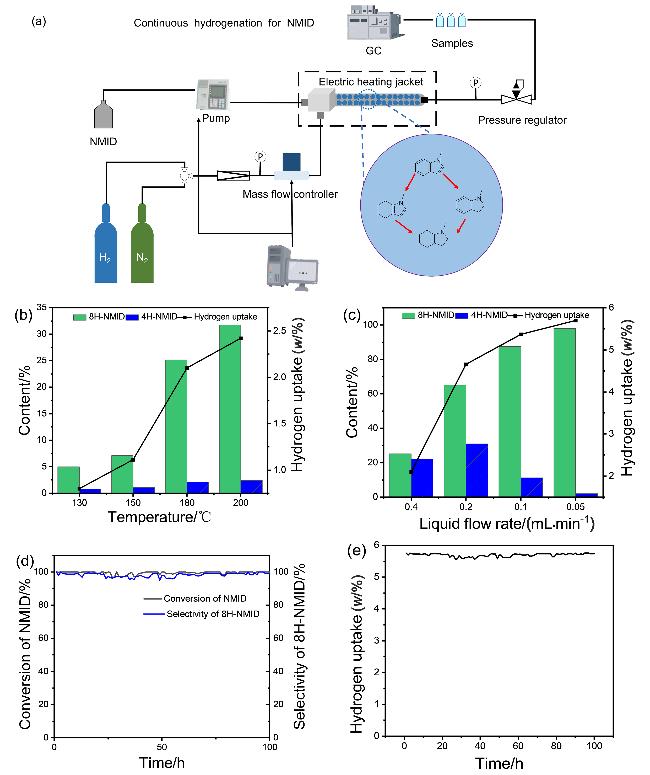
图6 (a)微填充床加氢NMID示意图; (b)反应温度对NMID连续加氢的影响(反应条件: 催化剂装填量: 6 g Ni2Al1-re450, 系统压力: 6 MPa; 气体流速: 100 mL/min; 液体流速: 0.4 mL/min); (c)液体流速对NMID的影响(反应条件: 催化剂装填量: 6 g Ni2Al1-re450; 系统压力: 6 MPa; 气体流速: 100 mL/min; 反应温度: 180 ℃). (d), (e) Ni2Al1- re450上NMID加氢的长期稳定性测试(反应条件: 反应温度: 180 ℃; 液体流速: 0.05 mL/min; 系统压力: 6 MPa; 气体流速: 100 mL/min; 装入催化剂量: 6 g Ni2Al1-re450)Figure 6 (a) Schematic diagram of hydrogenation NMID in Micro-packed bed reactor; (b) effect of reaction temperature on NMID continuous hydrogenation (reaction conditions: catalyst loading: 6 g Ni2Al1-re450; system pressure: 6 MPa; gas flow rate: 100 mL/min; liquid flow rate: 0.4 mL/min); (c) effect of liquid flow rate on NMID (reaction conditions: catalyst loading: 6 g Ni2Al1-re450; system pressure: 6 MPa; gas flow rate: 100 mL/min; reaction temperature: 180 ℃); (d), (e) long-term stability test for NMID hydrogenation over Ni2Al1-re450 (reaction conditions: reaction temperature: 180 ℃; gas flow rate: 100 mL/min; system pressure: 6 MPa; liquid flow rate: 0.05 mL/min; catalyst loading: 6 g Ni2Al1-re450) |
2.4 DFT计算
3 结论
4 实验部分
4.1 催化剂制备
4.2 催化剂表征
4.3 间歇式反应釜中氮甲基吲哚加氢性能测试
Ds=(Y×M×F)/W
Hydrogen uptake% (w)=(4MH2/M8H-NMI)Y8H-NMID
+(2MH2/M4H-NMID)×Y4H-NMID
TOF=nNMID×MNi/(nNi×Ds×t)