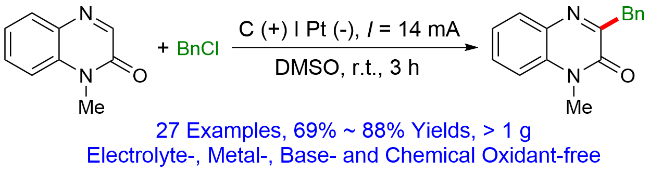
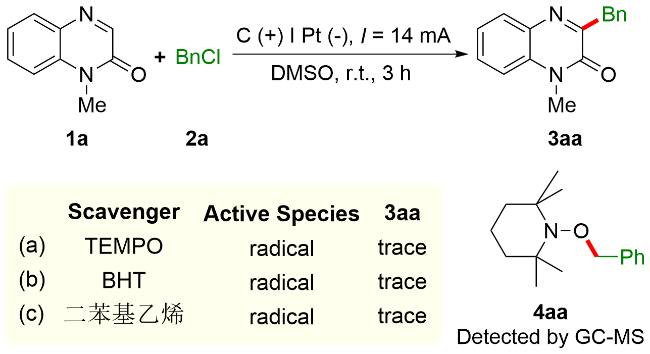
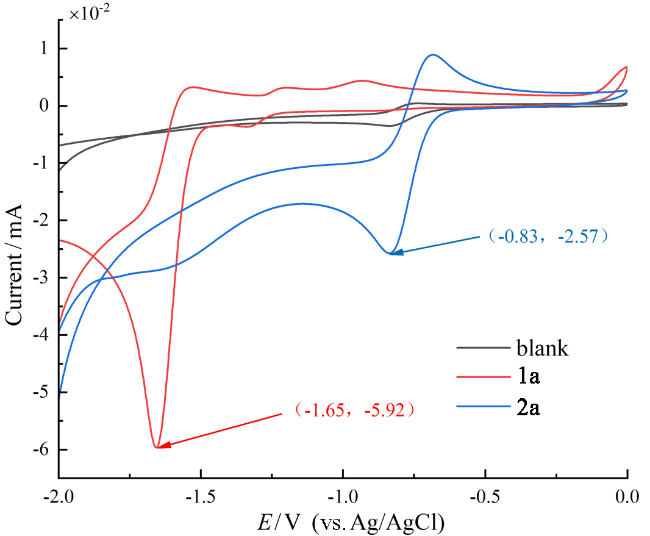
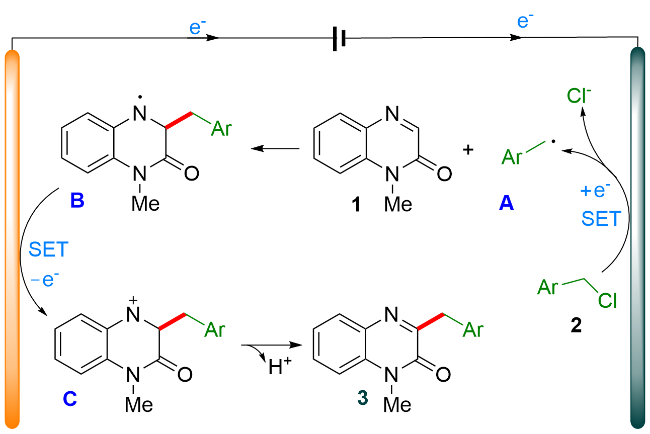
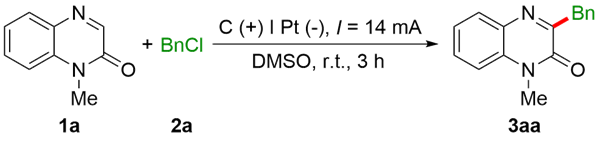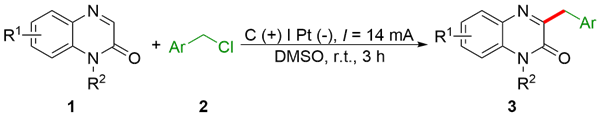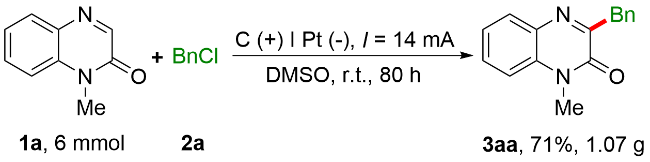1 引言
2 结果与讨论
2.1 无外加电解质参与的喹喔啉-2(1H)-酮和苄氯的电化学脱氯苄基化反应
2.1.1 苄基化反应条件的优化
表1 反应条件优化aTable 1 Optimization of reaction conditionsa |
| Entry | Variations from the standard condition | Yieldb/% | |||
|---|---|---|---|---|---|
| 1 | None | 88 | |||
| 2 | C(+)|C(-) instead of C(+)|Pt(-) | 59 | |||
| 3 | C(+)|Cu(-) instead of C(+)|Pt(-) | 38 | |||
| 4 | C(+)|Ni(-) instead of C(+)|Pt(-) | 33 | |||
| 5 | C(+)|Zn(-) instead of C(+)|Pt(-) | 21 | |||
| 6 | Pt(+)|Pt(-) instead of C(+)|Pt(-) | 53 | |||
| 7 | MeCN instead of DMSO | 24 | |||
| 8 | DCE, DMF, EtOH, EtOAc instead of DMSO | N.R. | |||
| 9 | 1 or 2 equiv. of 2a was used | 56, 88 | |||
| 10 | Under N2 | 88 | |||
| 11 | Without electricity | N.R. | |||
a Conditions: C (50 mm×5 mm×2 mm) as the anode, Pt (50 mm×5 mm×0.1 mm) as the cathode, constant current=14 mA, 1a (0.2 mmol), 2a (0.4 mmol), DMSO (3 mL), r.t., 3 h.; b Estimated by GC using dodecane as an internal reference. |
2.1.2 喹喔啉-2(1H)-酮的苄基化反应底物扩展
表2 3-苄基喹喔啉-2(1H)-酮底物范围aTable 2 Scope of C3-benzyl-quinoxalin-2(1H)-onea |
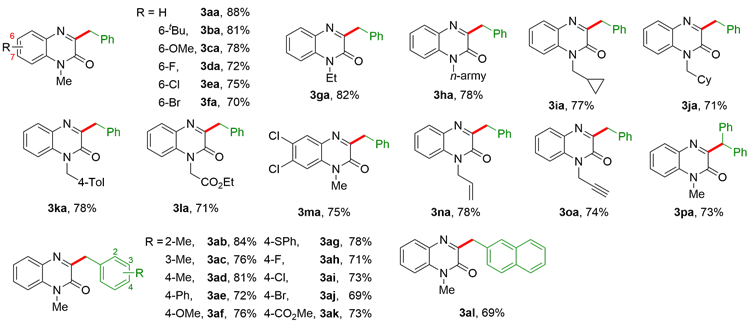 |
a Conditions: C (50 mm×5 mm×2 mm) as the anode, Pt (50 mm×5 mm×0.1 mm) as the cathode, constant current=14 mA, 1 (0.2 mmol), 2 (0.4 mmol), DMSO (3 mL), r.t., 3 h. |