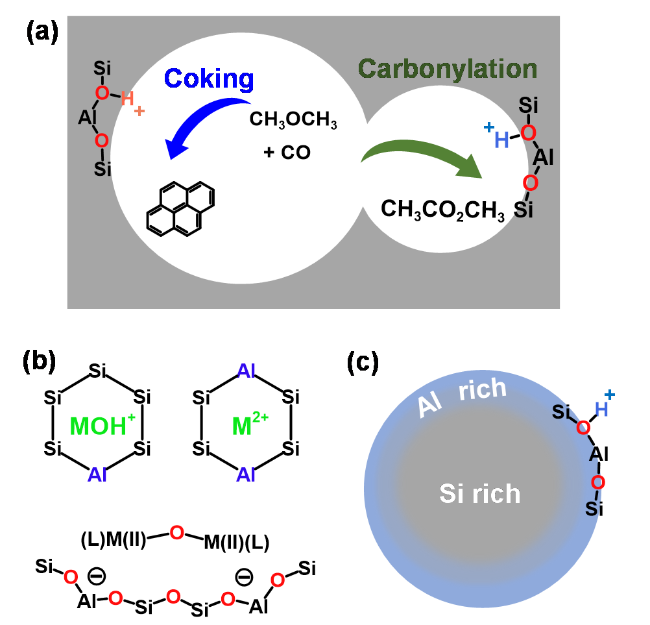
1 引言
图1 沸石不同孔道Al分布及反应选择性示意图(a), 不同Al分布(孤立Al, “Al对”)稳定金属离子类型及活性中间物种(b); 沸石晶粒内及表面Al的空间不均匀分布示意图(c)Figure 1 Schematic of zeolites with different channel Al distributions and reaction selectivity (a); types of metal ions stabilized by different Al distributions (isolated and paired Al) and active intermediate species (b); and spatial heterogeneity of Al distribution within and on the surface of zeolite crystals (c) |
2 沸石骨架Al分布调控策略
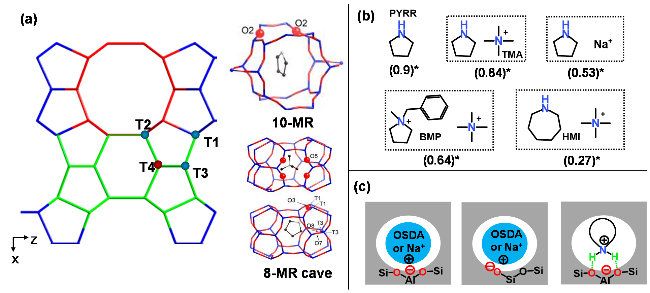
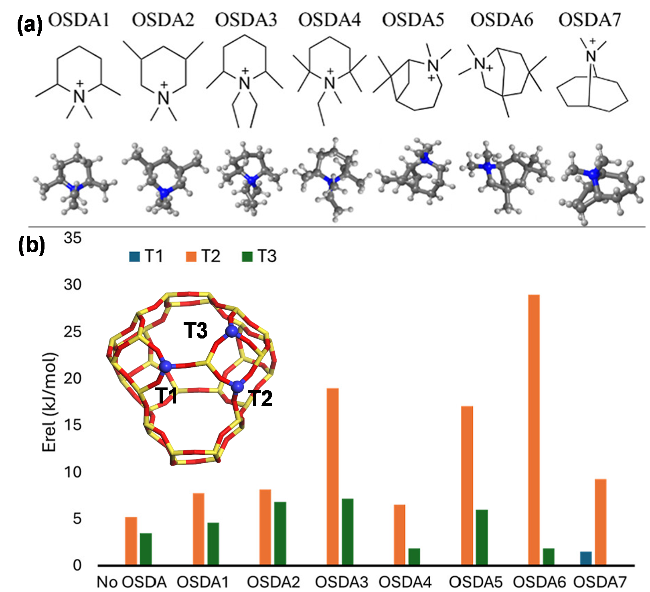
图2 FER沸石结构及典型有机结构导向剂分布示意图(a)[46], 用于合成FER沸石的不同结构导向剂及相应8元环孔穴酸位占比(b), 有机、无机结构导向剂与沸石骨架之间的不同作用示意图(c). *数据来自于文献[47-48]Figure 2 Schematic diagram of FER zeolite structure and distribution of typical OSDAs (a) (Adapted with permission from reference [46], Copyright 2013, American Chemical Society), different OSDAs used to synthesize FER zeolite and the corresponding proportion of acid sites on 8-membered ring cave annotated in parentheses (b), and schematic diagram of interactions between organic/inorganic SDAs and the framework of zeolite (c). * Data adapted from references [47-48] |
2.1 自下而上调控沸石中Al分布
2.1.1 有机(无机)结构导向剂调控沸石中Al分布
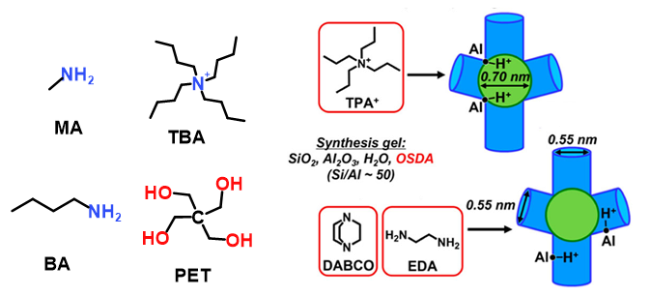
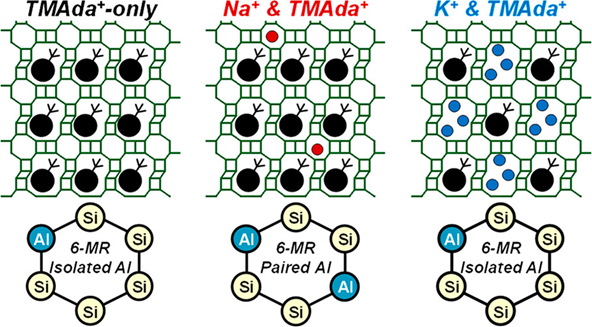
图3 合成ZSM-5沸石控制Al分布的常见结构导向剂及刚性季铵盐与柔性可成氢键有机胺导向不同Al分布示意图[63]Figure 3 Schematic draws of common SDAs used to synthesize ZSM-5 zeolite for controlling Al distribution, and the different Al distributions directed by rigid quaternary ammonium salts versus flexible, hydrogen-bond-capable organic amine. (Adapted with permission from reference [63], Copyright 2024, American Chemical Society) |
2.1.2 晶化条件的影响
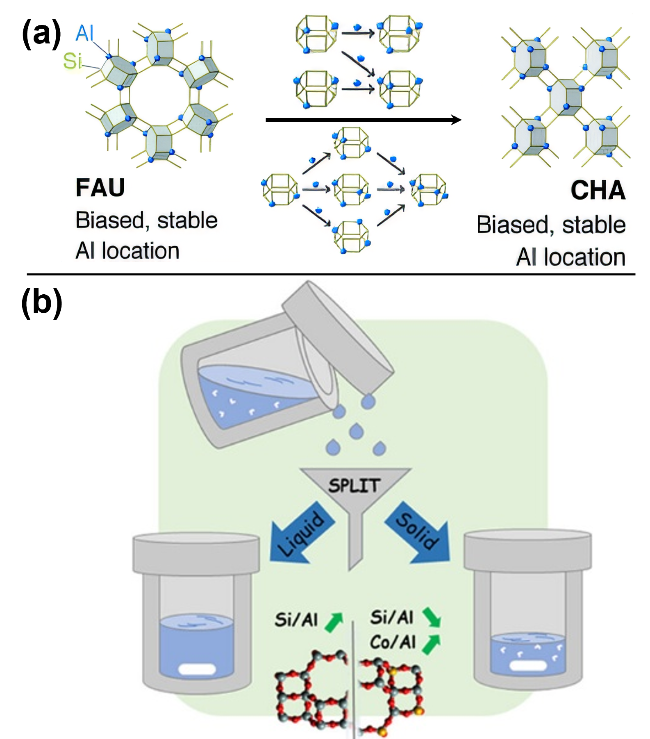
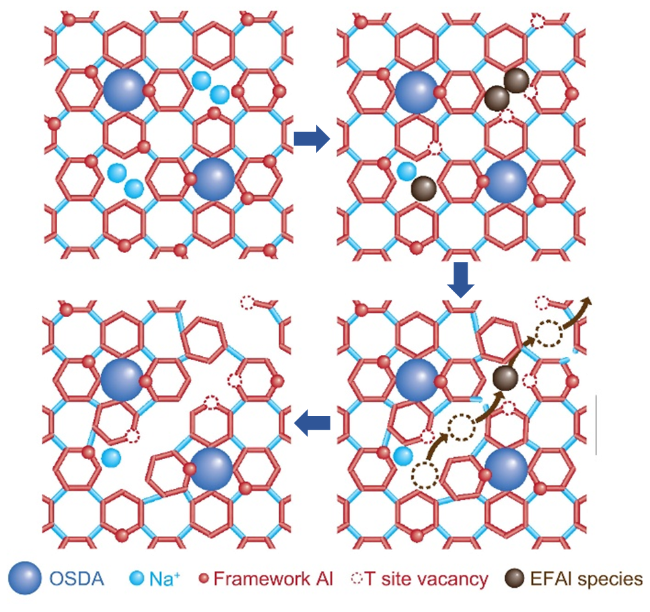
图6 FAU沸石转晶合成CHA沸石, FAU前驱体中的Al分布影响产物CHA沸石中Al分布的作用机制示意图(a)[96]及在FAU转晶合成CHA沸石所采用的分裂合成法示意图(b)[101]Figure 6 Schematic diagram illustrating (a) the mechanism of Al distribution in CHA zeolite product governed by FAU precursor during the FAU-to-CHA interzeolite conversion (IZC),[96] and (b) the split synthesis strategy used in the FAU-to-CHA IZC process[101] |
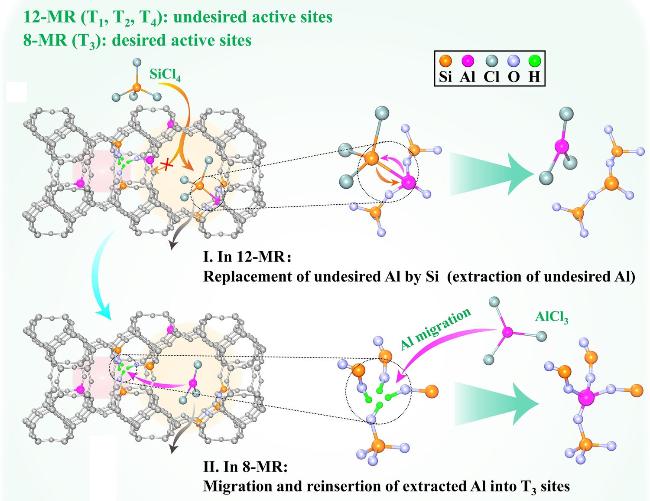
2.2 后处理选择性脱(补)Al策略
3 沸石Al分布的结构表征
3.1 27Al NMR研究沸石中不同Al位点
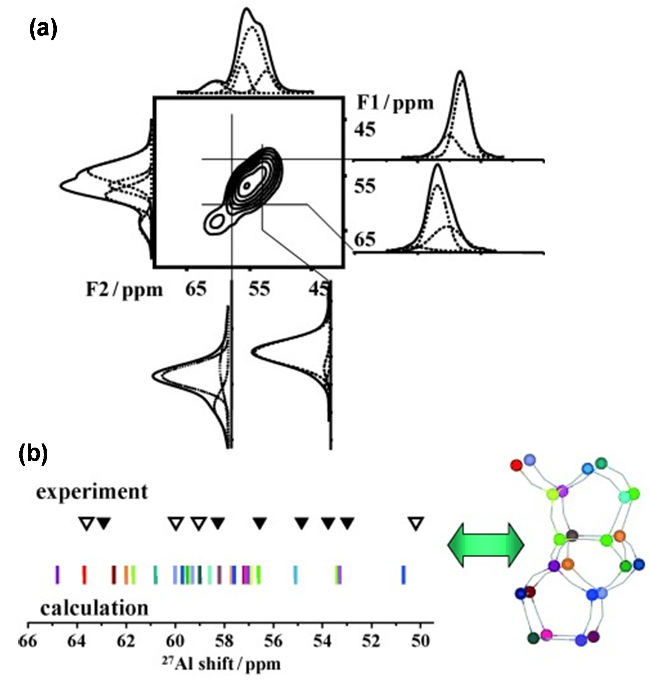
图9 27Al MQMAS NMR区分ZSM-5沸石中不同T位Al的27Al NMR信号(a), 相应实验所得27Al NMR化学位移与不同T位Al分布理论计算得到27Al NMR化学位移分布的对比图(b)[127]Figure 9 27Al MQMAS NMR distinguishing the 27Al NMR signals of different T-site Al in ZSM-5 zeolite (a), and comparison of the experimentally observed 27Al NMR chemical shifts with the distribution of 27Al NMR chemical shifts calculated from the theoretical distribution of Al among different T sites (b)[127] (Copyright 2007, Wiley-VCH GmbH) |
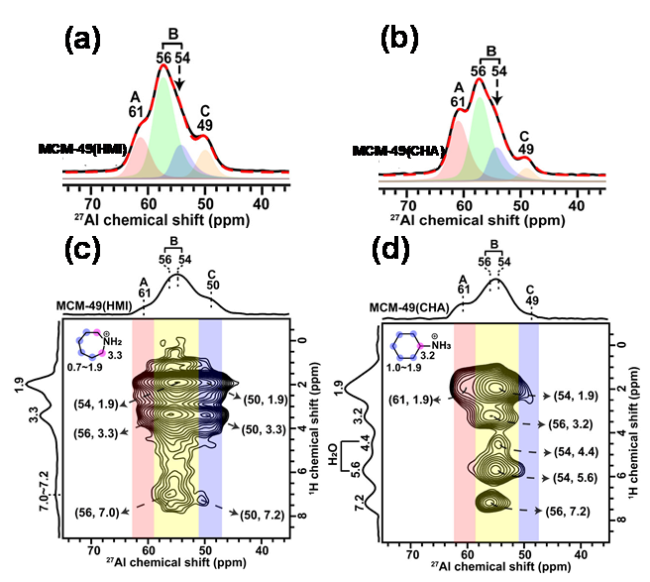
图10 以HMI和CHA为模板剂合成的MCM-49(HMI)和MCM-49(CHA)的27Al MAS NMR (a, b)及1H-27Al D-RINEPT NMR谱图(c, d)[135]Figure 10 27Al MAS NMR spectra (a, b) and 1H-27Al D-RINEPT spectra (c, d) of MCM-49 (HMI) and MCM-49 (CHA) synthesized using HMI and CHA as structure-directing agents. (Adapted with permission from reference [135], Copyright 2021, American Chemical Society) |
3.2 探针分子吸附结合X射线(中子)散射的结构精修
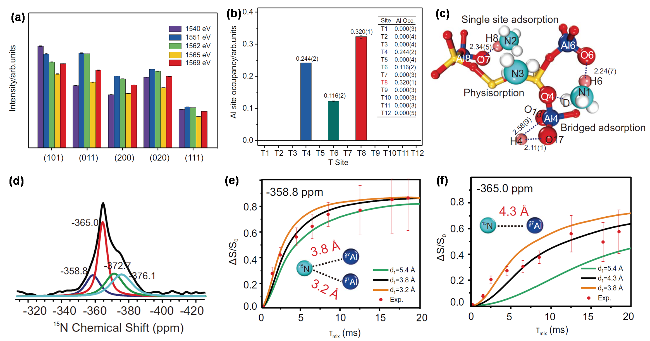
图11 软X射线在改变能量(共振、非共振)条件下, ZSM-5沸石的不同晶面散射强度变化(a), 由散射信号结构精修获得Al原子在不同T位的占有率(b), 中子散射获得ND3在ZSM-5沸石Brønsted酸位上的吸附构型图(c), 及利用15N MAS NMR (d) 15N-27Al S-RESPDOR (e~f)得到不同酸位点吸附的NH3及其与27Al核之间的距离[147]Figure 11 Soft X-ray scattering intensity changes of different ZSM-5 zeolite crystal facets under varying energy conditions (resonant, non-resonant) (a); Al atom site occupancies at different T-sites obtained by refinement of scattering signals (b); neutron scattering-derived adsorption configuration of ND3 on ZSM-5 zeolite Brønsted acid sites (c); and NH3 adsorbed on different acid sites and their distances to 27Al nuclei obtained using 15N MAS NMR (d) and 15N-27Al S-RESPDOR (e~f). (Reproduced from Guangchao Li et al., Science, doi:10.1126/science.adq6644 [2025], AAAS.) |