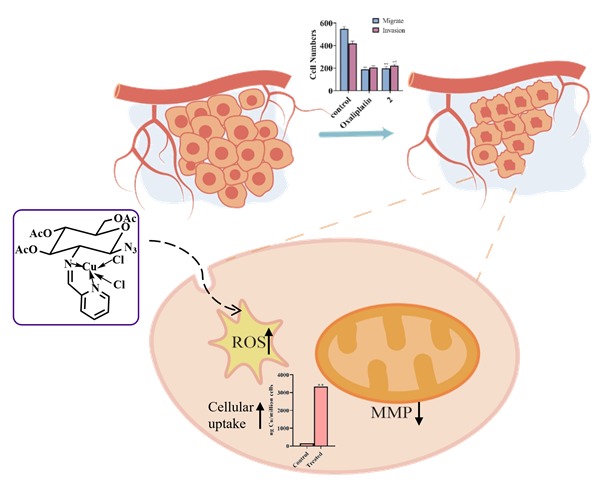
Introducing biologically active organic moieties into the coordination field of transition metal complexes has become an effective strategy for improving the selectivity and biocompatibility of metal drugs. In this paper, two ligands L1 and L2 with azidoacetylated glucosamine Schiff base structure were designed and synthesized, and then reacts with copper metal salts to synthesize three novel copper complexes. The single crystal structures of ligand L1 and complex 3 were analyzed by X-ray single crystal diffraction technology, and the in vitro cytotoxicity of the compounds on human malignant tumor cell lines and normal cell lines was determined by MTT assay. Among them, complex 2 showed high cytotoxicity against HepG-2 and HeLa cells, approaching or exceeding the value of the positive control, but the toxicity to normal cells was much lower than that of the positive control. The subsequent anti-tumor activity studies of complex 2 with strong cytotoxicity to tumor cells, including apoptosis assessment, intracellular reactive oxygen species generation, mitochondrial membrane potential, cell cycle, cell uptake, migration and invasion, and angiogenesis experiments, further confirmed its inhibitory mechanism on tumor cells. Due to the excellent water solubility of the sugar skeleton in the ligand, complex 2 exhibited good solubility and stability. We further demonstrated through intracellular reactive oxygen species (ROS) production, Hoechst 33342 staining experiment, mitochondrial membrane potential experiment, and Annexin V-mCherry that complex 2 can increase the level of ROS in cancer cells and induce apoptosis through the mitochondrial pathway Cell cycle experiments have shown that complex 2 can delay or inhibit the progression of the cell cycle through the S and G2/M phases. The significant cell uptake results indicated that the high cytotoxicity of complex 2 might be due to its extensive accumulation in HepG-2 cells. In addition, complex 2 can effectively inhibit the migration and invasion of cancer cells, as well as the angiogenesis of normal cell line umbilical vein endothelial cells (HUVEC). Molecular docking results showed that complex 2 has good binding ability with vascular endothelial growth factor receptor 2 (VEGFR-2).
[1] Alfonso-Herrera L. A.; Hernández-Romero D.; Cruz-Navarro J. A.; Ramos-Ligonio Á.; López-Monteon A.; Rivera-Villanueva J. M.; Morales-Morales D.; Colorado-Peralta, R. Coord. Chem. Rev.2024, 505, 215698.
[2] Roufosse B.; Serbu C.; Marschner C.; Prince S.; Blom, B. Eur. J. Med. Chem.2024, 274, 116528.
[3] Lv L.; Zheng T.; Tang L.; Wang Z.; Liu, W. Coord. Chem. Rev.2025, 525, 216327.
[4] Prathima T. S.; Choudhury B.; Ahmad M. G.; Chanda K.; Balamurali, M. M. Coord. Chem. Rev.2023, 490, 215231.
[5] Bortolamiol E.; Visentin F.; Scattolin T. Appl. Sci.-Basel2023, 13, 5561.
[6] Vaidya S. P.; Patra, M. Curr. Opin. Chem. Biol.2023, 72, 102236.
[7] Zhang Y.; Wu W.; Xu W.; Fu Y.; Guo H.; Lu, Z. Acta Chim. Sinica2025, 83, 445(in Chinese).
(张一亮, 武卫龙, 许文磊, 傅玉琴, 郭辉, 卢志强.化学学报, 2025, 83, 445.)
[8] McCallum, N.; Najlah, M.Cancers 2024, 16, 2775.
[9] Zahedipour F.; Dalirfardouei R.; Karimi G.; Jamialahmadi K. Biomed. Pharmacother.2017, 95, 1051.
[10] Song S.; Zhou H.; Li X.; Wang L.; Li Y.; Wang, J. Chinese J. Org. Chem.2014, 34, 706(in Chinese).
(宋沙沙,周宏勇,李小娜,王丽华, 李云庆, 王家喜, 氨基葡萄糖衍生物配体在不对称合成中的应用进展.有机化学, 2014, 34, 706.)
[11] Benessere V.; Del Litto R.; De Roma A.; Ruffo, F. Coord. Chem. Rev.2010, 254, 390.
[12] Benessere V.; De Roma A.; Del Litto R.; Lega M.; Ruffo, F. Eur. J. Org. Chem.2011, 2011, 5779.
[13] Sivaguru P.; Ning Y.; Bi X. Chem. Rev.2021, 121, 4253.
[14] Wang H.; Liu Y.; Xu M.; Cheng J. Biomater. Sci.2019, 7, 4166.
[15] Cheng B.; Wan Y.; Tang Q.; Du Y.; Xu F.; Huang Z.; Qin W.; Chen X.Chinese J. Chem. 2022, 40, 806.
[16] Sinicropi M. S.; Ceramella J.; Iacopetta D.; Catalano A.; Mariconda A.; Rosano C.; Saturnino C.; El-Kashef, H.; Longo, P.Int. J. Mol. Sci. 2022, 23, 14840.
[17] Salassa, L. Eur. J. Inorg. Chem.2011, 2011, 4931.
[18] Santini C.; Pellei M.; Gandin V.; Porchia M.; Tisato F.; Marzano C. Chem. Rev.2014, 114, 815.
[19] Wang C.; Yang X.; Dong C.; Chai K.; Ruan J.; Shi, S. Coord. Chem. Rev.2023, 487, 215156.
[20] Garcia-Peiro J. I.; Bonet-Aleta J.; Hueso, J. L. Coord. Chem. Rev.2025, 534, 216542.
[21] Zhang Y.-P.; Ma Z.-Y.; Qiao P.-P.; Gao C.-Y.; Tian J.-L.; Zhao J.-Z.; Du W.-J.; Xu J.-Y.; Yan, S.-P. J. Mol. Struct.2021, 1236, 130278.
[22] Addison A. W.; Rao T. N.; Reedijk J.; van Rijn J.; Verschoor, G. C. J. Chem. Soc., Dalton Trans.1984, 7, 1349.
[23] Bhattacharya S.; Karki S. S.; Suresh R.; Manikandan L.; Senthilkumar G. P.; Gupta M.; Mazumder U. K.; Balaji R.; Murali, K. Med. Chem. Res.2012, 21, 1120.
[24] Laggner H.; Hermann M.; Gmeiner B. M. K.; Kapiotis, S. Anal. Bioanal. Chem.2006, 385, 959.
[25] Henrotin Y. E.; Bruckner P.; Pujol, J. P. L. Osteoarthr. Cartilage2003, 11, 747.
[26] Chen Z.-P.; Li M.; Zhang L.-J.; He J.-Y.; Wu L.; Xiao Y.-Y.; Duan J.-A.; Cai T.; Li, W.-D. J. Drug Target.2015, 24, 492.
[27] Guo X.; Yang N.; Ji W.; Zhang H.; Dong X.; Zhou Z.; Li L.; Shen H. M.; Yao S. Q.; Huang W. Adv. Mater.2021, 33, 2007778.
[28] Zhang Y.-P.; He Q.; Zhou X.-H.; Liu G.-H.; Yue A.-Q.; Gao C.-Y.; Zhao J.-Z.; Du W.-J.; Yan, S.-P. J. Mol. Struct.2023, 1292, 136090.
[29] Zhang Y. P.; He Q.; Zhao Y. Y.; Qiao P. P.; Tian Y. W.; Gao C. Y.; Tian J. L.; Du W. J.; Yan, S. P. Appl. Organomet. Chem.2023, 37, e7074.
[30] Zhang Y.-P.; Tian Y.-W.; Geng J.; Zhou X.-H.; Li M.-Z.; Liu G.-H.; Gao C.-Y.; Yue A.-Q.; Zhao J.-Z.; Du W.-J.Arab. J. Chem. 2024, 17, 105478.
[31] Elefantova K.; Lakatos B.; Kubickova J.; Sulova Z.; Breier A.Int. J. Mol. Sci. 2018, 19, 1985.
[32] Song G.; Shang C.; Zhu Y.; Xiu Z.; Li Y.; Yang X.; Ge C.; Han J.; Jin N.; Li Y.; Li X.; Fang, J. Curr. Cancer Drug Tar.2024, 24, 411.
[33] Chen G.; Liao X.; Sun P.; Cen H.; Shu S.; Li B.; Li, J. Nan fang yi ke da xue xue bao = Journal of Southern Medical University2024, 44, 1109(in Chinese).
(陈桂玲, 廖晓凤, 孙鹏涛, 岑欢, 舒盛春, 李碧晶, 黎金华.南方医科大学学报, 2024, 44, 1109.)
[34] Khan H.; Alam W.; Alsharif K. F.; Aschner M.; Pervez S.; Saso L. Molecules2022, 27, 920.
[35] Gao F.; Xu J.-C.; You X.-R.; Gao X.; Wei J.-L.; Li S.-X.; Zhu C.-L.; Yang, C. Transl. Cancer Res.2019, 8, 203.
[36] Xu X.; Li S.; Lin Y.; Chen H.; Hu Z.; Mao Y.; Xu X.; Wu J.; Zhu Y.; Zheng X.; Luo J.; Xie, L. J. Transl. Med.2013, 11, 276.
[37] Zhang J.; Liu H.; Hou L.; Wang G.; Zhang R.; Huang Y.; Chen X.; Zhu J. Mol. Cancer2017, 16, 151.
[38] Zhang P. L.; Hou X. X.; Liu M. R.; Huang F. P.; Qin, X. Y. Dalton Trans.2020, 49, 6043.
[39] Kastl A.; Wilbuer A.; Merkel A. L.; Feng L.; Di Fazio P.; Ocker M.; Meggers E. Chem. Commun.2012, 48, 1863.
[40] Fante C.; Eldar-Boock A.; Satchi-Fainaro R.; Osborn H. M. I.; Greco, F. J. Med. Chem.2011, 54, 5255.
[41] Trott O.; Olson, A. J. J. Comput. Chem.2010, 31, 455.
[42] Peach C. J.; Mignone V. W.; Arruda M. A.; Alcobia D. C.; Hill S. J.; Kilpatrick L. E.; Woolard, J. International journal of molecular sciences2018, 19, 1264.
[43] Asha M. S.; Zabiulla; Khamees H. A.; Al-Ostoot F. H.; Pinto O.; Gopichand T.; Shivappaa, M. J. Mol. Struct.2022, 1253, 132272.
[44] Lega M.; Figliolia R.; Moberg C.; Ruffo F. Tetrahedron2013, 69, 4061.
[45] Tropper F. D.; Andersson F. O.; Braun S.; Roy R. Synthesis1992, 1992, 612.
[46] Mistri T.; Alam R.; Dolai M.; Mandal S. K.; Guha P.; Khuda-Bukhsh A. R.; Ali, M. Eur. J. Inorg. Chem.2013, 34, 5854.


