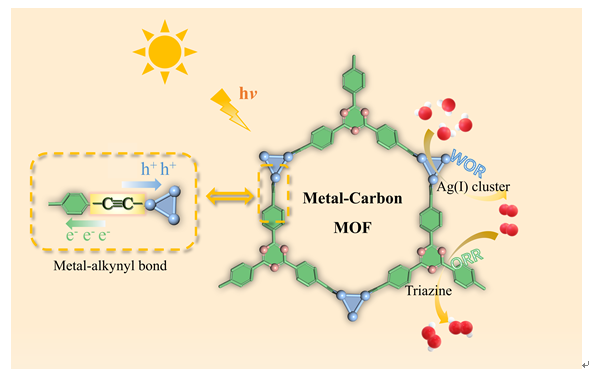
The development of highly efficient and stable photocatalysts for solar-driven hydrogen peroxide (H2O2) production represents a pivotal direction in green chemistry research. Herein, we report the successful synthesis of a novel silver-based metal-organic framework (MOF) photocatalyst, Ag-TEPT (where TEPT = 2,4,6-tris(4-ethynylphenyl)-1,3,5-triazine), which was constructed via a metal-carbon (M-C) bond coordination strategy to bridge Ag(I) clusters with triazine-based linkers. Under simulated sunlight irradiation, Ag-TEPT exhibited an outstanding H2O2 production rate of 2880 μmol g-1 h-1 in a pure water-oxygen system without any sacrificial agents, a performance that significantly surpasses most reported photocatalysts. Impressively, it maintained a high production rate of 2024 μmol g-1 h-1 even in ambient air. Furthermore, Ag-TEPT demonstrated remarkable photocatalytic stability with negligible activity loss over three consecutive reaction cycles. Post-catalytic characterization confirmed its unvaried crystal structure, morphology, and surface chemical states, attesting to its potential as a high-performance and durable photocatalyst. Mechanistic studies revealed a dual-pathway reaction mechanism, wherein H2O2 generation proceeds via simultaneous 2e- ORR and 4e- WOR processes. Notably, the O2 produced from the 4e- WOR serves as an internal feedstock for the 2e- ORR, mitigating the dependency on exogenous O2 and thereby enhancing the overall catalytic efficiency. In addition, the band structure of AgC-MOF, constructed from Tauc plot fitting and Mott-Schottky measurements, provided thermodynamic validation that Ag-TEPT is suitable for the photocatalytic reduction of O2 to H2O2 and the oxidation of H2O to O2, but not for the direct oxidation of H2O to H2O2. Photoluminescence spectroscopy and photoelectrochemical measurements confirmed the exceptional photogenerated charge separation efficiency of AgC-MOF, a benefit derived from the superior electron transport capability of the Ag-alkynyl bonds, which led to markedly improved photocatalytic reaction kinetics. In particular, Ag-TEPT showed a substantially higher photocurrent density, attributable to the excellent photosensitivity of the triazine units within the TEPT linker.
Chen Huiying
,
Huang Ningyu
,
Liao Peiqin
. A Novel Silver-Carbon Bond-Engineered Metal-Organic Framework toward Efficient Artificial Photosynthesis of Hydrogen Peroxide[J]. Acta Chimica Sinica, 0
: 20260106
-20260106
.
DOI: 10.6023/A25110383
[1] Yong Z.-J.;Ma, T.-Y. Angew. Chem. Int. Ed.2023, 62, e202308980.
[2] Shi X.-J.; Back S.; Gill T. M.; Siahrostami S.;Zheng X.-L. Chem.2021, 7, 38.
[3] Yi Y.-H.; Wang L.; Li G.;Guo, H.-C. Catal. Sci. Technol.2016, 6, 1593.
[4] Du M.-L.; Lu S.-L.; Dong W.-F.; Chen M.-Q.; Duan F.; Liu W.-H.;Yan, S.-R. Acta Chim. Sinica.2025, 83, 685.
[5] Ma D.; Xu M.; Ding L.; Guo J.-K.; Yang Y.; Dou W.; Li H.; Chen F.-J.;Tang Y.Angew. Chem. Int. Ed. 2025, doi: 10.1002/anie.202521029.
[6] Zuo Q.; Chu B.-X.; Ye X.-H.; Li F.-Y.; Li L.;Xu, Q. J. Am. Chem. Soc.2025, 147, 34681.
[7] Zeng X.-K.; Liu Y.; Hu X.-Y.;Zhang X.-W. Green Chem.2021, 23, 1466.
[8] Wu Q.-Y.; Zhang C.-X.; Sun K.;Jiang, H.-L. Acta Chim. Sinica.2020, 78, 688.
[9] Wang W.-J.; Chen D.; Li F.-Y.; Xiao X.;Xu Q. Chem.2024, 10, 86.
[10] Chen X.; Xia R. Q.; Deng Q. M.; Li Y. Q.; Chen M. H.; Li Y. G.; Cai X. C.; Titov A. A.; Filippov O. A.; Shubina E. S.; Wei R. J.; Ning G. H.;Li, D. Chin. J. Chem.2025, 43, 2277.
[11] Wang Y. X.; Zheng F.; Song D. X.; Niu B. Y.; Deng L. Q.;Zhang, X. M. Chin. J. Chem.2024, 42, 1093.
[12] Liu X.-G.; Shan Y.-Y.; Zhang S.-T.; Kong Q.-Q.;Pang, H. Green Energy Environ.2023, 8, 698.
[13] Shiraishi Y.; Kanazawa S.; Sugano Y.; Tsukamoto D.; Sakamoto H.; Ichikawa S.;Hirai T. ACS Catal.2014, 4, 774.
[14] Kondo Y.; Kuwahara Y.; Mori K.;Yamashita H. Chem.2022, 8, 2924.
[15] Moon G.; Kim W.; Bokare A. D.; Sung N.;Choi, W. Energy Environ. Sci.2014, 7, 4023.
[16] Sayed M.; Yu J.-G.; Liu G.;Jaroniec M. Chem. Rev.2022, 122, 10484.
[17] MATSUOKA M.; IINO K.; CHEN H.;ANPO, M. Res. Chem. Intermed.2004, 31, 153.
[18] Hui-Ying C.; Huang J. R.; Liu J. C.; Huang N. Y.; Chen X. M.;Liao, P. Q. Angew. Chem. Int. Ed.2024, 63, e202412553.
[19] Tang Y. Y.; Luo X.; Xia R. Q.; Luo J.; Peng S. K.; Liu Z. N.; Gao Q.; Xie M.; Wei R. J.; Ning G. H.;Li, D. Angew. Chem. Int. Ed.2024, 63, e202408186.
[20] Xia R. Q.; Liu Z. N.; Tang Y. Y.; Wu T.; Luo X.; Deng Q. M.; Ning G. H.;Li, D. Angew. Chem. Int. Ed.2025, 64, e202514091.
[21] Mohata S.; Majumder P.;Banerjee, R. Chem. Soc. Rev.2025, 54, 6062.
[22] Gong Z.-H.; Gao Y.; Li J.; Cai Z.-C.; Liu N.-F.;Jiang, J.-Z. Angew. Chem. Int. Ed.2025, 64, e202423205.
[23] Liu X.-Q.; Huang R.-R.; Peng L.-Y.; Yang J.-L.; Yan J.-B.; Zhai B.-B.; Luo Y.; Zhang C.; Tan S.-W.; Liu X.-Y.; Ding L.-P.;Fang, Y. Angew. Chem. Int. Ed.2024, 64, e202414472.
[24] Sun R.-X.; Yang X.-J.; Hu X.-L.; Guo Y.-T.; Zhang Y.-Q.; Shu C.; Yang X.; Gao H.; Wang X.-Y.; Hussain I.;Tan, B. Angew. Chem. Int. Ed.2024, 64, e202416350.
[25] Yang H.-Y.; Chen X.-K.; Mou Y.-J.; Li Q.; Liu J.-J.; Sun L.-J.; Zhai S.-L.; Deng W. Q.;Wu H. Small.2024, 20, 2406737.
[26] Chen H.-Y.; Zhao Z.-H.; Huang N.-Y.; Huang J.-R.;Liao, P.-Q. J. Am. Chem. Soc.2025, 147, 38484.
[27] Jiang L.; Lin L.; Wang Z.-H.; Ai H.-Y.; Jia J.-T.;Zhu, G.-S. J. Am. Chem. Soc.2024, 146, 22930.
[28] De Riggi N.; Imberdis A.; Nicolas E.;Cantat T. Organometallics.2024, 43, 2466.
[29] Sun Q.; Cai L.-L.; Ma H.-H.; Yuan C.-X.;Xu W. ACS Nano.2016, 10, 7023.
[30] Qin Y.-Y.; Wang Y.; Lu J.; Xu L.-L.;Wong, W. Y. Angew. Chem. Int. Ed.2024, 64, e202418269.
[31] Jiang L.; Jia J.-T.; Ma Y.-H.; Tian Y.-Y.; Zou X.-Q.;Zhu G.-S. Chem.2024, 10, 557.
[32] Liu J.; Chen Q.-W.; Cai K.; Li J.; Li Y.; Yang X.; Zhang Y.-J.; Wang Y.-F.; Tang H.; Zhao D.-H.;Wu K. Nat. Commun.2019, 10, 2545.
[33] Le´tinois-Halbes U.; Pale P.;Berger, S. J. Org. Chem.2005, 70, 9185.
[34] Feng Y.-L.; Wang G.-R.; Chang Y.; Cheng Y.; Sun B.-B.; Wang L.-M.; Chen C.-Y.;Zhang H.-Y. Nano Lett.2019, 19, 4478.
[35] Xu L.; Xiao Y.; Yu Z. X.; Yang Y.; Yan C.;Huang, J. Q. Angew. Chem. Int. Ed.2024, 63, e202406054.
[36] Wang C.; Wang S.-J.;Kong F.-G. Inorg. Chem.2021, 60, 5034.


