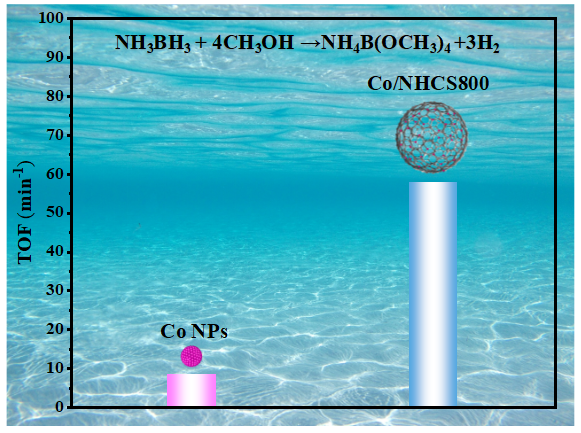
Nitrogen-doped hollow carbon materials are regarded as a highly promising catalyst support, owing to their structural advantages such as low density, high specific surface area, efficient mass transfer performance, and abundant nitrogen species, which collectively contribute to effectively stabilizing and dispersing metal active sites. In this work, for the first time, a nitrogen-doped hollow carbon spheres (NHCS) was synthesized by coating SiO2 nanospheres with a porphyrin-based organic frameworks (POFs) via a solvothermal method, followed by high-temperature pyrolysis and alkaline etching treatment. The influence of pyrolysis temperature on the physical and chemical properties of the material was systematically investigated. Results indicate that NHCS800, pyrolyzed at 800 °C, exhibited optimal structural stability and the most abundant surface defect sites. The Co/NHCS800 catalyst was prepared by uniformly dispersing cobalt nanoparticles on the support surface via a simple impregnation-reduction method. Characterization results revealed that the average size of the cobalt nanoparticles in the catalyst was approximately 2.5 nm, which was significantly smaller than that of cobalt particles formed in the absence of the support. For the catalytic dehydrogenation of ammonia borane (AB) via methanolysis, the Co/NHCS800 catalyst was ultrasonically dispersed in a single-neck round-bottom flask containing 5 mL of methanol. The flask was fixed in a constant-temperature water bath maintained at 25 °C. Once the temperature stabilized, 1.0 mmol of AB was rapidly added under vigorous stirring. The time required to generate every 5 mL of hydrogen gas was recorded until gas evolution ceased. The Co/NHCS800 catalyst demonstrated a remarkably high turnover frequency (TOF) value of 57.9 min⁻¹ for the methanolysis of ammonia borane. The catalysts were comprehensively characterized throughout the synthesis process using scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), Raman spectroscopy, and nitrogen adsorption-desorption measurements to monitor their morphological and structural evolution. The interaction between the metal and support was further investigated by X-ray photoelectron spectroscopy (XPS). The analysis indicates that the superior catalytic activity can be attributed to the large specific surface area of the support and the presence of ultrafine metal nanoparticles, as well as the strong electron transfer effect between nitrogen species and metal atoms.
[1] Schlapbach L.; Züttel A.Nature 2001, 414, 8234.
[2] Demirci U. B.; Miele, P. Energy Environ. Sci.2009, 2, 627.
[3] Turner J. A.Science 2004, 305, 972.
[4] Yadav M.; Xu Q.Energy Environ. Sci.2012, 5, 9698.
[5] Sakintuna B.;Lamari-Darkrim, F.; Hirscher, M. Int. J. Hydrogen Energy 2007, 32, 1121.
[6] David E.J. Mater.Process Technol. 2005, 162, 169.
[7] Umegaki T.; Yan J. M.; Zhang X. B.; Shioyama H.; Kuriyama N.; Xu Q.Int. J. Hydrogen Energy 2009, 34, 2303.
[8] Zhang A.; Yao Q.; Lu, Z. Acta Chim. Sinica,2021, 79, 885(in Chinese). (张安琪, 姚淇露, 卢章辉, 化学学报, 2021, 79, 885.)
[9] Liu Y.; Guan H.; Zhang J.; Zhao Y.; Yang J. H.; Zhang B.Int. J.Hydrogen Energy 2018, 43, 2754.
[10] Shi Y.; Xiang Z.; Deng J.; Nan J.; Zhang B. Mater. Lett.2019, 237, 61.
[11] Jiang H. L.; Xu Q. Catal. Today2011, 170, 56
[12] Demirci U. B.; Garin F.J. Alloys Compd. 2008, 463, 107.
[13] Feng Y.; Zhou X.; Yang J. H.; Gao X.; Yin L.; Zhao Y.; Zhang B.ACS Sustain. Chem. Eng. 2020, 8, 2122.
[14] Sun Q.; Wang N.; Bai R.; Hui Y.; Zhang T.; David A. D.; Zhang P.; Song L.; Miao S.; Yu J.Adv. Sci. 2019, 6, 1802350.
[15] Luo W.; Cheng W.; Hu M.; Wang Q.; Cheng X.; Zhang Y.; Wang Y.; Gao D.; Bi J.; Fan G.ChemSusChem. 2019, 12, 535.
[16] Li J.; Cai T.; Feng Y.; Liu X.; Wang N.; Sun, Q. Sci. China Chem.2024, 67, 2911.
[17] Çalışkan S.; Zahmakıran M.; Özkar, S. Appl. Catal. B: Environ.2010, 93, 387.
[18] Sun J. K.; Zhan W. W.; Akita T.; Xu Q.J. Am. Chem. Soc. 2015, 137, 7063.
[19] Özhava D.; Özkar S.Appl. Catal. B: Environ. 2016, 181, 716.
[20] Sun Q.; Wang N.; Zhang T.; Bai R.; Mayoral A.; Zhang P.; Zhang Q.; Terasaki O.; Yu, J. Angew. Chem. Int. Ed.2019, 58, 18570.
[21] Wang N.; Sun Q.; Zhang T.; Mayoral A.; Li L.; Zhou X.; Xu J.; Zhang P.; Yu, J. J. Am. Chem. Soc.2021, 143, 6905.
[22] Fang Y.; Li J. L.; Togo T.; Jin F. Y.; Xiao Z. F.; Liu L. J.; Drake H.; Lian X. Z.; Zhou H. C. Chem.2018, 4, 555.
[23] Guan S.; Yuan Z.; Zhao S.; Zhuang Z.; Zhang H.; Shen R.; Fan Y.; Li B.; Wang D.; Liu, B. Angew. Chem. Int. Ed.2024, 136, e202408193.
[24] Onat E.; Celik F. A.; Şahin Ö.; Karabulut E.; İZGİ, M. S. Chem. Eng. J.2024, 497, 154593.
[25] Wan C.; Li R.; Wang J.; Cheng D. G.; Chen F.; Xu L.; Gao M.; Kang Y.; Eguchi M.; Yamauchi, Y. Angew. Chem. Int. Ed.2024, 63, e202404505.
[26] Zhou A.; Cao Y.; Zhao N.; Jin Y.; Li Y.; Yang L.; Zhang C.; Gao Y.; Zhang Z.; Zhang Y.; Xie W.J. Am. Chem. Soc. 2024, 146, 33002
[27] Li J.; Feng Y.; Li X.; Zhang T.; Liu X.; Wang N.; Sun Q.ACS Catal. 2024, 14, 14665.
[28] Long J.; Wu H.; Liu Y.; Ding Y.; Yao Q.; Metin O.; Lu Z. cMat.2024, 1, e10.
[29] Li X.; Yao L.; Yao Q.; Xia J.; Lu, Z. H. Inorg. Chem. Front.,2025, 12, 5222.
[30] Li Y.; Jiang Y.; Jiang P.; Du S.; Jiang J.; Leng, Y. Acta. Chim. Sinica 2019, 77, 66(in Chinese). (李月姜, 宇晨, 蒋平平, 杜盛郁, 姜就胜, 冷炎, 化学学报, 2019, 77, 66.)
[31] Xu B.; Wei X.; Sun J.; Liu J.; Ma, L. Acta. Chim. Sinica 2023, 81, 239(in Chinese). (徐斌, 韦秀芝, 孙江敏, 刘建国, 马隆龙, 化学学报, 2023, 81, 293.)
[32] Zhang K.; Wang N.; Meng Y.; Zhang T.; Zhao P.; Sun Q.; Yu J. Chem. Sci.,2024, 15, 379.
[33] Li C.; He G.; Qu Z.; Zhang K.; Guo L.; Zhang T.; Zhang J.; Sun Q.; Mei D.; Yu, J. Angew. Chem. Int. Ed.2024, 63, e202409001.
[34] Zhao M.; Wang X.; Xu J.; Li Y.; Wang X.; Chu X.; Wang K.; Wang Z.; Zhang L.; Feng J.; Song S.; Zhang H. Adv. Mater.2024, 36, 2313596.
[35] Yao, Q. L; He, M.; Kong Y. R.; Gui T.; Lu, Z. H. Rare Metals 2023, 42, 3410.
[36] Tian S.; Bai R.; Gao Z.; Chen Z.; Wang M.; Tang H.; Lin S.; Xu B.; Liu X.; Yu J.; Ma D. J. Am. Chem. Soc. 2025, 147, 30268.
[37] Jayaprakash N.; Shen J.; Moganty S. S.; Corona A. A.L. A.; Archer, L. A.Angew. Chem. Int. Ed. 2011, 50, 5904.
[38] Yuan C.; Guo Z.; Lou D. X.W.; Zhang, C.; Wu, H. B. Angew. Chem. Int. Ed. 2012, 51, 9592.
[39] He G.; Evers S.; Liang X.; Cuisinier M.; Garsuch A.; Nazar, L. F. ACS Nano.2013, 7, 10920.
[40] Zhang R. P.; Li W. C.; Hao G. P.; Lu, A. H. Nano. Res.2021, 14, 315.
[41] Zhou H.; Cui Q.; Hu X.; Yang W.; Tian X.; Wang S.Acta. Chim. Sinica 2024, 82, 503(in Chinese). (周何鑫, 崔青云, 胡雪敏, 杨文秀, 田肖, 王硕, 化学学报, 2024, 82, 503.
[42] Li X.Ph.D. Dissertation. Jiangxi Normal University, Nanchang, 2022 (in Chinese).(李修刚, 博士论文, 江西师范大学, 南昌, 2022.)
[43] Cui X.; Long Y.; Zhou X.; Yu G.; Yang J.; Yuan M.; Ma J.; Dong Z. Green Chem.2018, 20, 1121.
[44] Zhang R.; Gu X.; Liu Y.; Hua D.; Shao M.; Gu Z.; Wu J.; Zheng B.; Zhang W.; Li S.; Huo F.; Huang W. Appl. Surf. Sci. 2020, 512, 145740.
[45] Xie C.; Yan D.; Li H.; Du S.; Chen W.; Wang Y.; Zhou Y.; Chen R.; Wang S. ACS Catal,2020, 10, 11082.
[46] Fang X.; Liu S.; Zang J.; Xu C.; Zheng M. S.; Dong Q. F.; Sun D.; Zheng N.Nanoscale 2013, 5, 6908.
[47] Kuhn P.; Antonietti M.; Thomas A. Angew. Chem. Int. Ed. 2008, 47, 3450.


