Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (3): 903-926.DOI: 10.6023/cjoc202310024 Previous Articles Next Articles
Special Issue: 光电催化综述合集
REVIEWS
收稿日期:2023-10-24
修回日期:2023-11-21
发布日期:2024-04-02
基金资助:
Xinyue Fang, Yawen Huang, Xinwei Hu( ), Zhixiong Ruan(
), Zhixiong Ruan( )
)
Received:2023-10-24
Revised:2023-11-21
Published:2024-04-02
Contact:
*E-mail: xinweihu@gzhmu.edu.cn; zruan@gzhmu.edu.cn
Supported by:Share
Xinyue Fang, Yawen Huang, Xinwei Hu, Zhixiong Ruan. Recent Progress in Electrochemical Modification of Amino Acids and Peptides[J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 903-926.

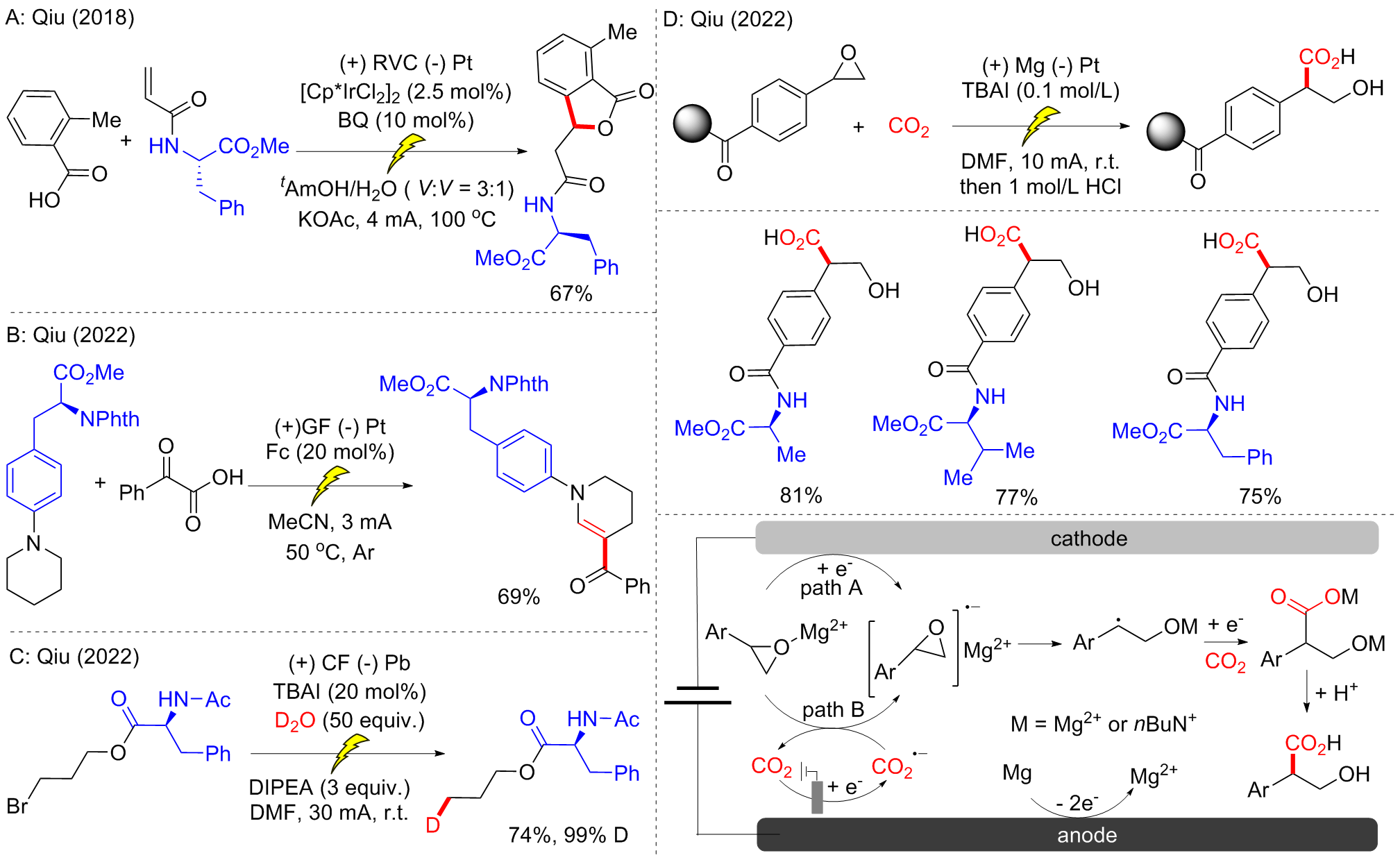
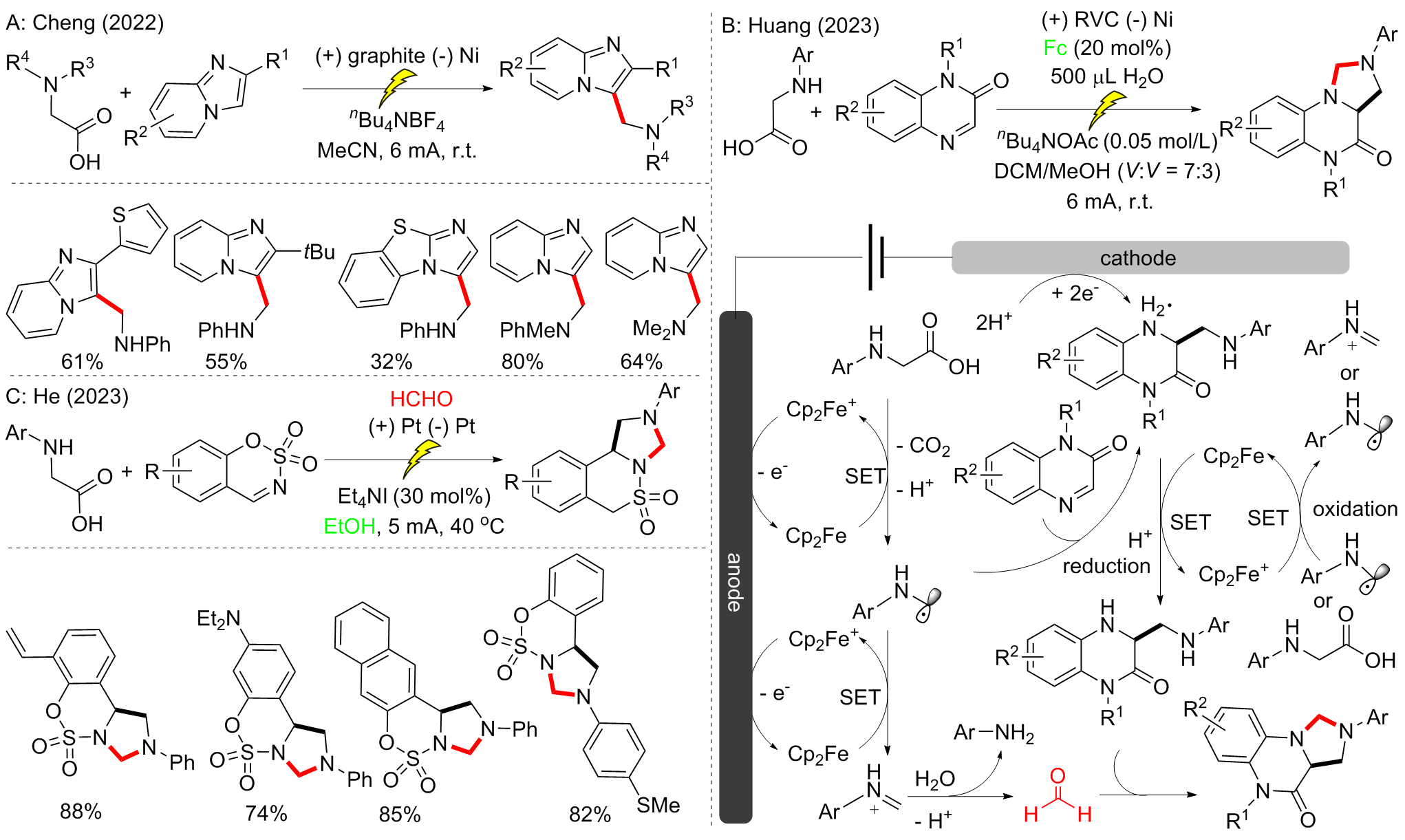
| [1] |
Lau J. L.; Dunn M. K. Bioorg. Med. Chem. 2018, 26, 2700.
doi: 10.1016/j.bmc.2017.06.052 |
| [2] |
(a) Craik D. J.; Fairlie D. P.; Liras S.; Price D. Chem. Biol. Drug Des. 2013, 81, 136.
doi: 10.1111/cbdd.2012.81.issue-1 pmid: 33536635 |
|
(b) Cooper B. M.; Iegre J.; O'Donovan D. H.; Halvarsson M. Ö.; Spring D. R. Chem. Soc. Rev. 2021, 50, 1480.
doi: 10.1039/D0CS00556H pmid: 33536635 |
|
|
(c) Muttenthaler M.; King G. F.; Adams D. J.; Alewood P. F. Nat. Rev. Drug Discov. 2021, 20, 309.
doi: 10.1038/s41573-020-00135-8 pmid: 33536635 |
|
|
(d) McCarver S. J.; Qiao J. X.; Carpenter J.; Borzilleri R. M.; Poss M. A.; Eastgate M. D.; Miller M. M.; MacMillan D. W. C. Angew. Chem., Int. Ed. 2017, 56, 728.
doi: 10.1002/anie.v56.3 pmid: 33536635 |
|
| [3] |
Fosgerau K.; Hoffmann T. Drug Discovery Today 2015, 20, 122.
doi: 10.1016/j.drudis.2014.10.003 pmid: 25450771 |
| [4] |
(a) Ball Z. T. Acc. Chem. Res. 2013, 46, 560.
doi: 10.1021/ar300261h pmid: 31318534 |
|
(b) Yi L.; Sun H.; Wu Y. W.; Triola G.; Waldmann H.; Goody R. S. Angew. Chem., Int. Ed. 2010, 122, 9607.
doi: 10.1002/ange.v122.49 pmid: 31318534 |
|
|
(c) deGruyter J. N.; Malins L. R.; Baran P. S. Biochemistry 2017, 56, 3863.
doi: 10.1021/acs.biochem.7b00536 pmid: 31318534 |
|
|
(d) Cravatt B. F.; Wright A. T.; Kozarich J. W. Annu. Rev. Biochem. 2008, 77, 383.
doi: 10.1146/biochem.2008.77.issue-1 pmid: 31318534 |
|
|
(e) Luther A.; Bisang C.; Obrecht D. Bioorg. Med. Chem. 2018, 26, 2850.
doi: 10.1016/j.bmc.2017.08.006 pmid: 31318534 |
|
|
(f) Bai Z.; Wang H. Synlett 2020, 31, 199.
doi: 10.1055/s-0039-1691495 pmid: 31318534 |
|
|
(g) Brimble M. A.; Zhang S.;Rodriguez, L. M. M. D. L.; Li, F. F. Chem. Sci. 2023, 14, 7782.
doi: 10.1039/D3SC02543H pmid: 31318534 |
|
|
(h) Budisa N.; Völler J.; Koksch B.; Acevedo-Rocha C.; Kubyshkin V.; Agostini F. Angew. Chem., Int. Ed. 2017, 56, 9680.
doi: 10.1002/anie.v56.33 pmid: 31318534 |
|
|
(i) Chow H. Y.; Zhang Y.; Matheson E.; Li X. Chem. Rev. 2019, 119, 9971.
doi: 10.1021/acs.chemrev.8b00657 pmid: 31318534 |
|
|
(j) King T. A.; Kandemir J. M.; Walsh S. J.; Spring D. R. Chem. Soc. Rev. 2021, 50, 39.
doi: 10.1039/D0CS00344A pmid: 31318534 |
|
|
(k) Liu J.; Wang P.; Yan Z.; Yan J.; Kenry; Zhu Q. ChemBioChem 2021, 22, 2762.
doi: 10.1002/cbic.v22.18 pmid: 31318534 |
|
|
(l) Mondal S.; Chowdhury S. Adv. Synth. Catal. 2018, 360, 1884.
doi: 10.1002/adsc.v360.10 pmid: 31318534 |
|
| [5] |
Bottecchia C.; Noël T. Chem.-Eur. J. 2018, 25, 26.
doi: 10.1002/chem.v25.1 |
| [6] |
Noisier A. F.; Brimble M. A. Chem. Rev. 2014, 114, 8775.
doi: 10.1021/cr500200x pmid: 25144592 |
| [7] |
Wang W.; Lorion M. M.; Shah J.; Kapdi A. R.; Ackermann L. Angew. Chem., Int. Ed. 2018, 57, 14700.
doi: 10.1002/anie.v57.45 |
| [8] |
Tong H.-R.; Li B.; Li G.; He G.; Chen G. CCS Chem. 2021, 3, 1797.
doi: 10.31635/ccschem.020.202000426 |
| [9] |
Meyer T. H.; Choi I.; Tian C.; Ackermann L. Chem 2020, 6, 2484.
doi: 10.1016/j.chempr.2020.08.025 |
| [10] |
Siu J. C.; Fu N.; Lin S. Acc. Chem. Res. 2020, 53, 547.
doi: 10.1021/acs.accounts.9b00529 |
| [11] |
Yamamoto K.; Kuriyama M.; Onomura O. Acc. Chem. Res. 2019, 53, 105.
doi: 10.1021/acs.accounts.9b00513 |
| [12] |
Ang N. W.; Oliveira J. C.; Ackermann L. Angew. Chem., Int. Ed. 2020, 59, 12842.
doi: 10.1002/anie.v59.31 |
| [13] |
Rosen B.; Werner E. J. Am. Chem. Soc. 2014, 136, 5571.
doi: 10.1021/ja5013323 |
| [14] |
Mackay A. S.; Payne R. J.; Malins L. R. J. Am. Chem. Soc. 2021, 144, 23.
doi: 10.1021/jacs.1c11185 pmid: 34968405 |
| [15] |
Brabec V.; Mornstein V. Biophys. Chem. 1980, 12, 159.
pmid: 17000148 |
| [16] |
(a) Brunelle P.; Rauk A. J. Phys. Chem. 2004, 108, 11032.
doi: 10.1021/jp046626j pmid: 4865316 |
|
(b) Harriman A. J. Phys. Chem. 1987, 91, 6102.
doi: 10.1021/j100308a011 pmid: 4865316 |
|
|
(c) Jocelyn P. Eur. J. Biochem. 1967, 2, 327.
pmid: 4865316 |
|
|
(d) Navaratnam S.; Parsons B. J. Chem. Soc., Faraday Trans. 1998, 94, 2577.
pmid: 4865316 |
|
|
(e) Wilson G. S.; Glass R. S. J. Inorg. Biochem. 1994, 55, 87.
doi: 10.1016/0162-0134(94)85031-3 pmid: 4865316 |
|
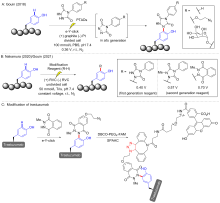
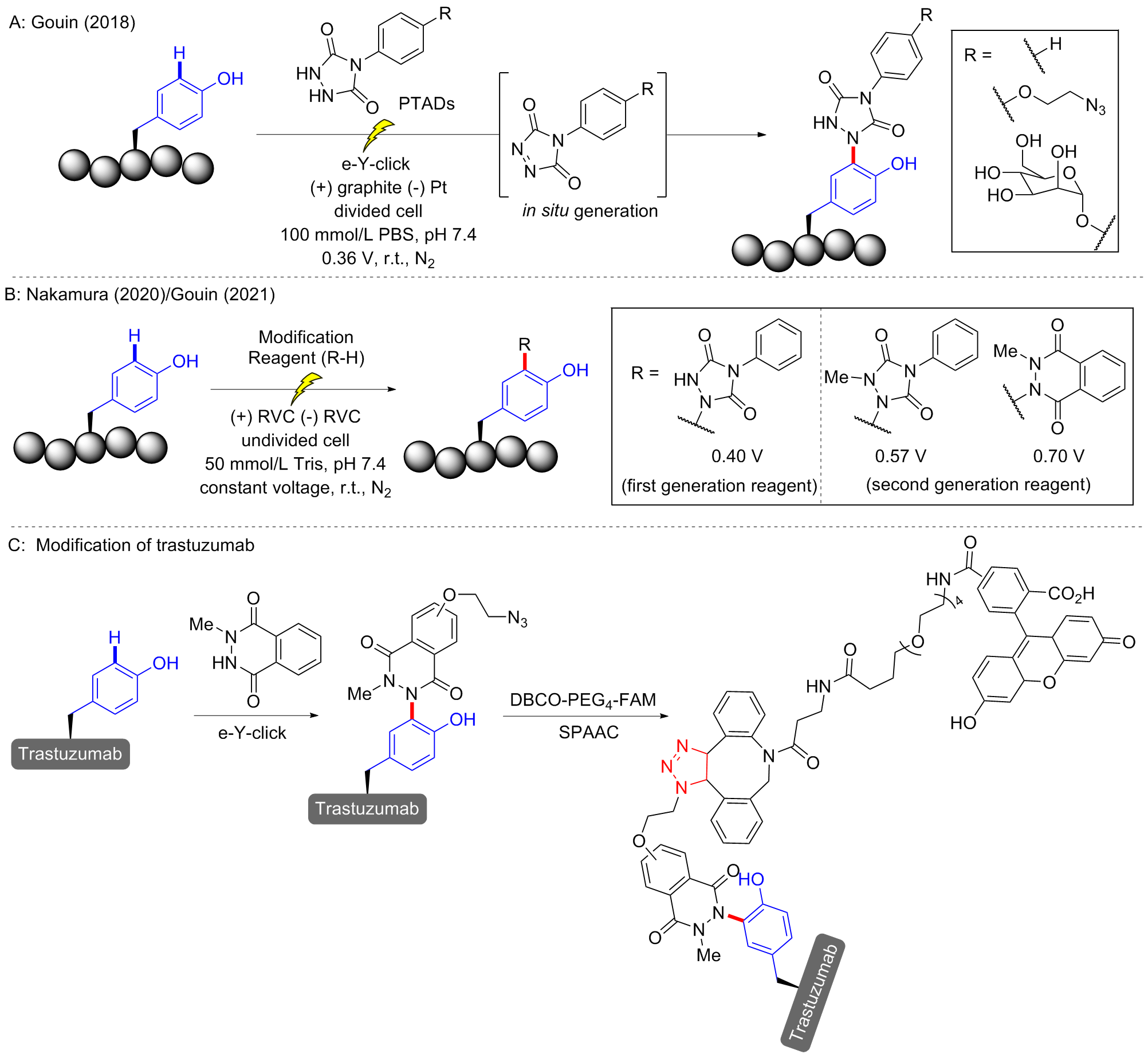
| [17] |
Alvarez-Dorta D.; Thobie-Gautier C.; Croyal M.; Bouzelha M.; Mével M.; Deniaud D.; Boujtita M.; Gouin S. G. J. Am. Chem. Soc. 2018, 140, 17120.
doi: 10.1021/jacs.8b09372 pmid: 30422648 |
| [18] |
Ban H.; Gavrilyuk J.; Barbas III C. F. J. Am. Chem. Soc. 2010, 132, 1523.
doi: 10.1021/ja909062q |
| [19] |
Ban H.; Nagano M.; Gavrilyuk J.; Hakamata W.; Inokuma T.; Barbas III C. F. Bioconjugate Chem. 2013, 24, 520.
doi: 10.1021/bc300665t |
| [20] |
Jessica F.; Corentin W.; Sylvestre D.; Christian L.; André L. RSC Adv. 2013, 3, 24936.
doi: 10.1039/c3ra44666b |
| [21] |
(a) Nilo A.; Allan M.; Brogioni B.; Proietti D.; Cattaneo V.; Crotti S.; Sokup S.; Zhai H.; Margarit I.; Berti F. Bioconjugate Chem. 2014, 25, 2105.
doi: 10.1021/bc500438h |
|
(b) Hu Q.-Y.; Allan M.; Adamo R.; Quinn D.; Zhai H.; Wu G.; Clark K.; Zhou J.; Ortiz S.; Wang B. Chem. Sci. 2013, 4, 3827.
doi: 10.1039/c3sc51694f |
|
| [22] |
Bauer D. M.; Ahmed I.; Vigovskaya A.; Fruk L. Bioconjugate Chem. 2013, 24, 1094.
doi: 10.1021/bc4001799 |
| [23] |
Madl C. M.; Heilshorn S. C. Bioconjugate Chem. 2017, 28, 724.
doi: 10.1021/acs.bioconjchem.6b00720 |
| [24] |
Cui L.; Ma Y.; Li M.; Wei Z.; Huan Y.; Li H.; Fei Q.; Zheng L. Anal. Chem. 2021, 93, 4434.
doi: 10.1021/acs.analchem.0c04337 |
| [25] |
Sato, S.; Matsumura, M.; Kadonosono, T.; Abe, S.; Ueno, T.; Ueda, H.; Nakamura, H. Bioconjugate Chem. 2020, 31, 1417.
doi: 10.1021/acs.bioconjchem.0c00120 |
| [26] |
Depienne S.; Alvarez-Dorta D.; Croyal M.; Temgoua R. C. T.; Charlier C.; Deniaud D.; Mével M.; Boujtita M.; Gouin S. G. Chem. Sci. 2021, 12, 15374.
doi: 10.1039/D1SC04809K |
| [27] |
Song C.; Liu K.; Wang Z.; Ding B.; Wang S.; Weng Y.; Chiang C.-W.; Lei A. Chem. Sci. 2019, 10, 7982.
doi: 10.1039/C9SC02218J |
| [28] |
Stangier M.; Messinis A. M.; Oliveira J. C. A.; Yu H.; Ackermann L. Nat. Commun. 2021, 12, 4736.
doi: 10.1038/s41467-021-25005-8 |
| [29] |
Hou X.; Kaplaneris N.; Yuan B.; Frey J.; Ohyama T.; Messinis A. M.; Ackermann L. Chem. Sci. 2022, 13, 3461.
doi: 10.1039/D1SC07267F |
| [30] |
You S.; Wang R.; Ma C.; Lu C.; Yang G.; Liu L.; Weng Y.; Gao M. Org. Chem. Front. 2023, 10, 4606.
doi: 10.1039/D3QO00983A |
| [31] |
Toyama E.; Maruyama K.; Sugai T.; Kondo M.; Masaoka S.; Saitoh T.; Oisaki K.; Kanai M. 2019, 10.26434/ chemrxiv.7795484.v1.
|
| [32] |
Seki Y.; Ishiyama T.; Sasaki D.; Abe J.; Sohma Y.; Oisaki K.; Kanai M. J. Am. Chem. Soc. 2016, 138, 10798.
doi: 10.1021/jacs.6b06692 |
| [33] |
Wu J.; Abou-Hamdan H.; Guillot R.; Kouklovsky C.; Vincent G. Chem. Commun. 2020, 56, 1713.
doi: 10.1039/C9CC09276E |
| [34] |
Weng Y.; Xu X.; Chen H.; Zhang Y.; Zhuo X. Angew. Chem., Int. Ed. 2022, 61, e202206308.
doi: 10.1002/anie.v61.41 |
| [35] |
Qiu Y.; Scheremetjew A.; Finger L. H.; Ackermann L. Chem.- Eur. J. 2020, 26, 3241.
doi: 10.1002/chem.v26.15 |
| [36] |
Chen H. C.; Wan C.; Shih W. H.; Kao C. Y.; Jiang H.; Weng Y.; Chiang C. W. Asian J. Org. Chem. 2022, 12, e202200647.
doi: 10.1002/ajoc.v12.1 |
| [37] |
Kawamata Y.; Vantourout J. C.; Hickey D. P.; Bai P.; Chen L.; Hou Q.; Qiao W.; Barman K.; Edwards M. A.; Garrido-Castro A. F.; deGruyter J. N.; Nakamura H.; Knouse K.; Qin C.; Clay K. J.; Bao D.; Li C.; Starr J. T.; Garcia-Irizarry C.; Sach N.; White H. S.; Neurock M.; Minteer S. D.; Baran P. S. J. Am. Chem. Soc. 2019, 141, 6392.
doi: 10.1021/jacs.9b01886 pmid: 30905151 |
| [38] |
Ma Y.; Hong J.; Yao X.; Liu C.; Zhang L.; Fu Y.; Sun M.; Cheng R.; Li Z.; Ye J. Org. Lett. 2021, 23, 9387.
doi: 10.1021/acs.orglett.1c03500 |
| [39] |
Novaes L. F. T.; Ho J. S. K.; Mao K.; Liu K.; Tanwar M.; Neurock M.; Villemure E.; Terrett J. A.; Lin S. J. Am. Chem. Soc. 2022, 144, 1187.
doi: 10.1021/jacs.1c09412 pmid: 35015533 |
| [40] |
Lamb C. M. G.; Shi J.; Wilden J. D.; Macmillan D. Org. Biomol. Chem. 2022, 20, 7343.
doi: 10.1039/D2OB01499H |
| [41] |
Mackay A. S.; Maxwell J. W.; Bedding M. J.; Kulkarni S. S.; Byrne S. A.; Kambanis L.; Popescu M. V.; Paton R. S.; Malins L. R.; Ashhurst A. S.; Corcilius L.; Payne R. J. Angew. Chem.,Int. Ed. 2023, e202313037.
|
| [42] |
You S.; Ruan M.; Lu C.; Liu L.; Weng Y.; Yang G.; Wang S.; Alhumade H.; Lei A.; Gao M. Chem. Sci. 2022, 13, 2310.
doi: 10.1039/D1SC06757E |
| [43] |
Liu L.; Xu Z.; Liu T.; Xu C.; Zhang W.; Hua X.; Ling F.; Zhong W. J. Org. Chem. 2022, 87, 11379.
doi: 10.1021/acs.joc.2c00856 |
| [44] |
Wang R.; Wang J.; Zhang Y.; Wang B.; Xia Y.; Xue F.; Jin W.; Liu C. Adv. Synth. Catal. 2023, 365, 900.
doi: 10.1002/adsc.v365.6 |
| [45] |
Kawamata Y.; Hayashi K.; Carlson E.; Shaji S.; Waldmann D.; Simmons B. J.; Edwards J. T.; Zapf C. W.; Saito M.; Baran P. S. J. Am. Chem. Soc. 2021, 143, 16580.
doi: 10.1021/jacs.1c06572 pmid: 34596395 |
| [46] |
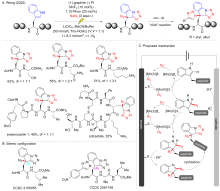
Zeng S.; Fang S.; Cai H.; Wang D.; Liu W.; Hu X.; Sun P.; Ruan Z. Chem. Asian J. 2022, 17, e202200762.
doi: 10.1002/asia.v17.20 |
| [47] |
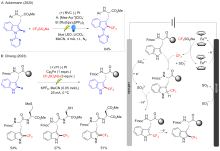
Qiu Y.; Stangier M.; Meyer T. H.; Oliveira J. C. A.; Ackermann L. Angew. Chem., Int. Ed. 2018, 57, 14179.
|
| [48] |
Feng T.; Wang S.; Liu Y.; Liu S.; Qiu Y. Angew. Chem., Int. Ed. 2021, 61, e202115178.
doi: 10.1002/anie.v61.6 |
| [49] |
Li P.; Guo C.; Wang S.; Ma D.; Feng T.; Wang Y.; Qiu Y. Nat. Commun. 2022, 13, 3774.
doi: 10.1038/s41467-022-31435-9 |
| [50] |
Wang Y.; Tang S.; Yang G.; Wang S.; Ma D.; Qiu Y. Angew. Chem., Int. Ed. 2022, 61, e202207746.
doi: 10.1002/anie.v61.38 |
| [51] |
Liang H.; Julaiti Y.; Zhao C.-G.; Xie J. Nat. Synth. 2023, 2, 338.
doi: 10.1038/s44160-022-00219-w |
| [52] |
Huang H.; Lambert T. H. Angew. Chem., Int. Ed. 2021, 60, 11163.
doi: 10.1002/anie.v60.20 |
| [53] |
Huang H.; Lambert T. H. J. Am. Chem. Soc. 2021, 143, 7247.
doi: 10.1021/jacs.1c01967 pmid: 33949852 |
| [54] |
Klocke E.; Matzeit A.; Gockeln M.; Schäfer H. J. Chem. Ber. 1993, 126, 1623.
doi: 10.1002/cber.v126:7 |
| [55] |
Shao X.; Zheng Y.; Tian L.; Martín-Torres I.; Echavarren A. M.; Wang Y. Org. Lett. 2019, 21, 9262.
doi: 10.1021/acs.orglett.9b03696 |
| [56] |
Chen X.; Luo X.; Peng X.; Guo J.; Zai J.; Wang P. Chem. Eur. J. 2020, 26, 3226.
doi: 10.1002/chem.v26.15 |
| [57] |
Barton L. M.; Chen L.; Blackmond D. G.; Baran P. S. Proc. Natl. Acad. Sci. 2021, 118, e2109408118.
doi: 10.1073/pnas.2109408118 |
| [58] |
Qin T.; Malins L. R.; Edwards J. T.; Merchant R. R.; Novak A. J.; Zhong J. Z.; Mills R. B.; Yan M.; Yuan C.; Eastgate M. D. Angew. Chem., Int. Ed. 2017, 56, 260.
doi: 10.1002/anie.v56.1 |
| [59] |
McCarver S. J.; Qiao J. X.; Carpenter J.; Borzilleri R. M.; Poss M. A.; Eastgate M. D.; Miller M. M.; MacMillan D. W. Angew. Chem., Int. Ed. 2017, 129, 746.
doi: 10.1002/ange.v129.3 |
| [60] |
Köckinger M.; Hanselmann P.; Roberge D. M.; Geotti-Bianchini P.; Kappe C. O.; Cantillo D. Green Chem. 2021, 23, 2382.
doi: 10.1039/D1GC00201E |
| [61] |
Renaud P.; Seebach D. Angew. Chem.,Int. Ed. Engl. 1986, 25, 843.
doi: 10.1002/anie.v25:9 |
| [62] |
Lin Y.; Malins L. R. Chem. Sci. 2020, 11, 10752.
doi: 10.1039/D0SC03701J |
| [63] |
Lin Y.; Malins L. R. J. Am. Chem. Soc. 2021, 143, 11811.
doi: 10.1021/jacs.1c05718 |
| [64] |
Li S.; Li X.; Wang T.; Yang Q.; Ouyang Z.; Chen J.; Zhai H.; Li X.; Cheng B. Adv. Synth. Catal. 2022, 364, 2346.
doi: 10.1002/adsc.v364.14 |
| [65] |
Cui J.-F.; Zhong W.-Q.; Huang J.-M. J. Org. Chem. 2023, 88, 1147.
doi: 10.1021/acs.joc.2c02654 |
| [66] |
Lu Y.-H.; Mu S.-Y.; Li H.-X.; Jiang J.; Wu C.; Zhou M.-H.; Ouyang W.-T.; He W.-M. Green Chem. 2023, 25, 5539.
doi: 10.1039/D2GC04906F |
| [67] |
Gausmann M.; Kreidt N.; Christmann M. Org. Lett. 2023, 25, 2228.
doi: 10.1021/acs.orglett.3c00403 |
| [68] |
Li Y.; Wang H.; Zhang H.; Lei A. Chin. J. Chem. 2021, 39, 3023.
doi: 10.1002/cjoc.v39.11 |
| [69] |
Li C.-J. Acc. Chem. Res. 2009, 42, 335.
doi: 10.1021/ar800164n |
| [70] |
Wang H.; He M.; Li Y.; Zhang H.; Yang D.; Nagasaka M.; Lv Z.; Guan Z.; Cao Y.; Gong F.; Zhou Z.; Zhu J.; Samanta S.; Chowdhury A. D.; Lei A. J. Am. Chem. Soc. 2021, 143, 3628.
doi: 10.1021/jacs.1c00288 |
| [71] |
Palma A.; Cárdenas J.; Frontana-Uribe B. A. Green Chem. 2009, 11, 283.
doi: 10.1039/B815745F |
| [72] |
Nagahara S.; Okada Y.; Kitano Y.; Chiba K. Chem. Sci. 2021, 12, 12911.
doi: 10.1039/d1sc03023j pmid: 34745521 |
| [73] |
Chiba K.; Kono Y.; Kim S.; Nishimoto K.; Kitano Y.; Tada M. Chem. Commun. 2002, No. 16, 1766.
|
| [74] |
(a) Cortes-Clerget M.; Berthon J.-Y.; Krolikiewicz-Renimel I.; Chaisemartin L.; Lipshutz B. H. Green Chem. 2017, 19, 4263.
doi: 10.1039/C7GC01575E pmid: 34270883 |
|
(b) Cortes-Clerget M.; Spink S. E.; Gallagher G. P.; Chaisemartin L.; Filaire E.; Berthon J.-Y.; Lipshutz B. H. Green Chem. 2019, 21, 2610.
doi: 10.1039/c9gc01050e pmid: 34270883 |
|
|
(c) Knauer S.; Koch N.; Uth C.; Meusinger R.; Avrutina O.; Kolmar H. Angew. Chem., Int. Ed. 2020, 59, 12984.
doi: 10.1002/anie.v59.31 pmid: 34270883 |
|
|
(d) Pawlas J.; Rasmussen J. H. ChemSusChem. 2021, 14, 3231.
doi: 10.1002/cssc.202101028 pmid: 34270883 |
| [1] | Liangming Xuan, Wei Zhao, Rundong Fan, Qiongjiao Yan, Wei Wang, Fen'er Chen. Recent Advances of the Catalysis Systems in the α-C(sp3)—H Functionalization of Glycine Derivatives [J]. Chinese Journal of Organic Chemistry, 2024, 44(9): 2700-2721. |
| [2] | Lan Zhou, Hong He, De-Qiao Yang, Zhong-Wei Hou, Lei Wang. Electrochemical Trifluoromethylation/Spirocyclization of N-Benzylacrylamides to Construct Trifluoromethylated 2-Azaspiro[4.5]decanes [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 981-988. |
| [3] | Jian Huang, Wenzhen Zhang. Advances in Electrochemical Cathodic Reductive Reactions Involving Carbon-Nitrogen Bonds [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 825-839. |
| [4] | Haoruo Shangguan, Ping Huang, Zhenya Dai, Ping Wang. Synthesis and Application of β-Thiolated Amino Acids [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3089-3097. |
| [5] | Wanjie Wei, Lei Zhan, Lei Gao, Guobao Huang, Xianli Ma. Research Progress of Electrochemical Synthesis of C-Sulfonyl Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 17-35. |
| [6] | Nana Wang, Jingcheng Xu, Haibo Mei, Hiroki Moriwaki, Kunisuke Izawa, Vadim A. Soloshonok, Jianlin Han. Electrochemical Approaches for Preparation of Tailor-Made Amino Acids [J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3034-3049. |
| [7] | Zeyin Meng, Chengtao Feng, Kun Xu. Recent Advances in the Electrochemical Formation of Carbon-Nitrogen Bonds [J]. Chinese Journal of Organic Chemistry, 2021, 41(7): 2535-2570. |
| [8] | Guang Yang, Yanwei Wang, Youai Qiu. Advances in Organic Photoelectrochemical Synergistic Catalysis [J]. Chinese Journal of Organic Chemistry, 2021, 41(10): 3935-3947. |
| [9] | Wu Ping, Ma Chunhua, Xu Senhan, Li Yingxia, Zhai Yanjun, Zhang Wei. Facile Synthesis of (S)-4-Amino-5-mercaptopentanoic Acid [J]. Chin. J. Org. Chem., 2016, 36(1): 191-195. |
| [10] | Wang Yingjie, Zhang Zhenfeng, Zhang Wanbin. Asymmetric Hydrogenation of Cyclic Dehydroamino Acids and Their Derivatives [J]. Chin. J. Org. Chem., 2015, 35(3): 528-538. |
| [11] | Li Xiaona, Zhou Hongyong, Zhang Pengliang, Wang Jiaxi. Synthesis of Chiral Amino-acids Derivatives:Silica-gel Promoted Ring Opening of Substituted Aziridines [J]. Chin. J. Org. Chem., 2013, 33(12): 2545-2550. |
| [12] | SUN Xiao-Min, ZHANG Wei, DING Ning, WANG Peng, LI Ying-Xia. Progress in the Synthesis of One Kind of α-Heterocycle Five-Membered Amino Acids [J]. Chin. J. Org. Chem., 2011, 31(10): 1563-1572. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||