Chinese Journal of Organic Chemistry ›› 2025, Vol. 45 ›› Issue (3): 951-958.DOI: 10.6023/cjoc202410001 Previous Articles Next Articles
ARTICLES
收稿日期:2024-10-02
修回日期:2024-11-07
发布日期:2024-12-19
作者简介:基金资助:
Weidong Dong, Haiyun Liu, Xinyu Li, Biao Wang, Bo Cheng, Suwei Dong( )
)
Received:2024-10-02
Revised:2024-11-07
Published:2024-12-19
Contact:
* E-mail: About author:Supported by:Share
Weidong Dong, Haiyun Liu, Xinyu Li, Biao Wang, Bo Cheng, Suwei Dong. Studies toward Chemical Synthesis of Homogeneously Glycosylated Interleukin-10[J]. Chinese Journal of Organic Chemistry, 2025, 45(3): 951-958.
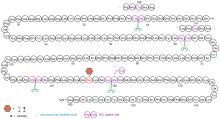

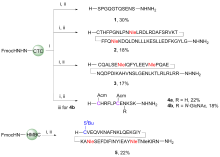

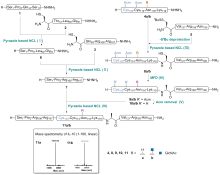

| Entry | Folding redox (conc.) | Buffer | Process | Resultsb |
|---|---|---|---|---|
| 1[ | GSH/GSSG (2/0.2 mmol/L) | 6 mol/L Gnd•HCl, 5 mmol/L dithiothreitol (DTT); then 50 mmol/L Tris-HCl, 50 mmol/ L NaCl, 5 mmol/L EDTA, pH 8.0 | 2~8 ℃, 16 h | Complete aggregation |
| 2[ | Cysteine/Cystine (4/0.5 mmol/L) | 100 mmol/L Tris-HCl, pH 8.5, Gnd•HCl from 6 mol/L to 3 mol/L to 1 mol/L | Stepwise dialysis, 48 h in total | Complete aggregation |
| 3[ | Cysteine/Cystamine (5/1 mmol/L) | 10 mmol/L DTT, 6 mol/L Gnd•HCl, 100 mmol/L NaCl, 5 mmol/L ethylenediamine- tetraacetic acid (EDTA), 20 mmol/L Tris•HCl; then 500 mmol/L Gnd•HCI, 20 mmol/ L Tris, 15% Glycerol, 1 mol/L Arginine, pH 9.0 | 2~8 ℃, 72 h | Products with 4 less of molecular weight than that of the reduced forms |
| Entry | Folding redox (conc.) | Buffer | Process | Resultsb |
|---|---|---|---|---|
| 1[ | GSH/GSSG (2/0.2 mmol/L) | 6 mol/L Gnd•HCl, 5 mmol/L dithiothreitol (DTT); then 50 mmol/L Tris-HCl, 50 mmol/ L NaCl, 5 mmol/L EDTA, pH 8.0 | 2~8 ℃, 16 h | Complete aggregation |
| 2[ | Cysteine/Cystine (4/0.5 mmol/L) | 100 mmol/L Tris-HCl, pH 8.5, Gnd•HCl from 6 mol/L to 3 mol/L to 1 mol/L | Stepwise dialysis, 48 h in total | Complete aggregation |
| 3[ | Cysteine/Cystamine (5/1 mmol/L) | 10 mmol/L DTT, 6 mol/L Gnd•HCl, 100 mmol/L NaCl, 5 mmol/L ethylenediamine- tetraacetic acid (EDTA), 20 mmol/L Tris•HCl; then 500 mmol/L Gnd•HCI, 20 mmol/ L Tris, 15% Glycerol, 1 mol/L Arginine, pH 9.0 | 2~8 ℃, 72 h | Products with 4 less of molecular weight than that of the reduced forms |


| [1] |
(a) Fiorentino, D. F.; Bond, M. W.; Mosmann, T. R. J. Exp. Med. 1989, 170, 2081.
|
|
(b) Chaudhry, A.; Samstein, R. M.; Treuting, P.; Liang, Y.; Pils, M. C.; Heinrich, J.-M.; Jack, R. S.; Wunderlich, F. T.; Brüning, J. C.; Müller, W.; Rudensky, A. Y. Immunity 2011, 34, 566.
|
|
|
(c) Murai, M.; Turovskaya, O.; Kim, G.; Madan, R.; Karp, C. L.; Cheroutre, H.; Kronenberg, M. Nat. Immunol. 2009, 10, 1178.
|
|
| [2] |
(a) Yoon, S. I.; Logsdon, N. J.; Sheikh, F.; Donnelly, R. P.; Walter, M. R. Biol. Chem. 2006, 281, 35088.
|
|
(b) Murray, P. J. Curr. Opin. Pharmacol. 2006, 6, 379.
|
|
| [3] |
Kühn, R.; Löhler, J.; Rennick, D.; Rajewsky, K.; Müller, W. Cell 1993, 75, 263.
|
| [4] |
Couper, K. N.; Blount, D. G.; Riley, E. M. J. Immunol. 2008, 180, 5771.
|
| [5] |
Burmeister, A. R.; Marriott, I. Front. Cell. Neurosci. 2018, 12, 458.
|
| [6] |
Rajbhandari, P.; Thomas, B. J.; Feng, A.-C.; Hong, C.; Wang, J.; Vergnes, L.; Sallam, T.; Wang, B.; Sandhu, J.; Seldin, M. M.; Lusis, A. J.; Fong, L. G.; Katz, M.; Lee, R.; Young, S. G.; Reue, K.; Smale, S. T.; Tontonoz, P. Cell 2018, 172, 218.
|
| [7] |
Quiros, M.; Nishio, H.; Neumann, P. A.; Siuda, D.; Brazil, J. C.; Azcutia, V.; Hilgarth, R.; O'Leary, M. N.; Garcia-Hernandez, V.; Leoni, G. J. Clin. Invest. 2017, 127, 3510.
|
| [8] |
King, A.; Balaji, S.; Le, L. D.; Crombleholme, T. M.; Keswani, S. G. Adv. Wound Care 2014, 3, 315.
|
| [9] |
Wang, X.; Wong, K.; Ouyang, W.; Rutz, S. Cold Spring Harbor Perspect. Biol. 2019, 11, a028548.
|
| [10] |
Vieira, P.; de Waal-Malefyt, R.; Dang, M. N.; Johnson, K. E.; Kastelein, R.; Fiorentino, D. F.; de Vries, J. E.; Roncarolo, M. G.; Mosmann, T. R.; Moore, K. W. Proc. Natl. Acad. Sci. U. S. A. 1991, 88, 1172.
|
| [11] |
Zdanov, A.; Schalk-Hihi, C.; Gustchina, A.; Tsang, M.; Weatherbee, J.; Wlodawer, A. Structure 1995, 3, 591.
|
| [12] |
Logsdon, N. J.; Jones, B. C.; Allman, J. C.; Izotova, L.; Schwartz, B.; Pestka, S.; Walter, M. R. J. Mol. Biol. 2004, 342, 503.
|
| [13] |
Josephson, K.; DiGiacomo, R.; Indelicato, S. R.; Iyo, A. H.; Nagabhushan, T. L.; Parker, M. H.; Walter, M. R. J. Biol. Chem. 2000, 275, 13552.
|
| [14] |
(a) Windsor, W. T.; Syto, R.; Tsarbopoulos, A.; Zhang, R.; Durkin, J.; Baldwin, S.; Paliwal, S.; Mui, P. W.; Pramanik, B.; Trotta, P. P. Biochemistry 1993, 32, 8807.
|
|
(b) Moore, K. W.; de Waal Malefyt, R.; Coffman, R. L.; O'Garra, A. Annu. Rev. Immunol. 2001, 19, 683.
|
|
| [15] |
Moore, K. W.; Vieira, P.; Fiorentino, D. F.; Trounstine, M. L.; Khan, T. A.; Mosmann, T. R. Science 1990, 248, 1230.
|
| [16] |
(a) Wang, P.; Dong, S.; Shieh, J.-H.; Peguero, E.; Hendrickson, R.; Moore, M. A. S.; Danishefsky, S. J. Science 2013, 342, 1357.
|
|
(b) Murakami, M.; Kiuchi, T.; Nishihara, M.; Tezuka, K.; Okamoto, R.; Izumi, M.; Kajihara, Y. Sci. Adv. 2016, 2, e1500678.
|
|
| [17] |
Reif, A.; Lam, K.; Weidler, S.; Lott, M.; Boos, I.; Lokau, J.; Bretscher, C.; Mönnich, M.; Perkams, L.; Schmälzlein, M.; Graf, C.; Fischer, J.-P.; Lechner, C.; Hallstein, K.; Becker, S.; Weyand, M.; Steegborn, C.; Schultheiss, G.; Rose-John, S.; Garbers, C.; Unverzagt, C. Angew. Chem., Int. Ed. 2021, 60, 13380.
|
| [18] |
Sakamoto, I.; Tezuka, K.; Fukae, K.; Ishii, K.; Taduru, K.; Maeda, M.; Ouchi, M.; Yoshida, K.; Nambu, Y.; Igarashi, J.; Hayashi, N.; Tsuji, T.; Kajihara, Y. J. Am. Chem. Soc. 2012, 134, 5428.
|
| [19] |
Li, H.; Zhang, J.; An, C.; Dong, S. J. Am. Chem. Soc. 2021, 143, 2846.
|
| [20] |
Dong, W.; Yang, X.; Li, X.; Wei, S.; An, C.; Zhang, J.; Shi, X.; Dong, S. J. Am. Chem. Soc. 2024, 146, 18270.
|
| [21] |
Zhao, J.; Liu, X.; Liu, J.; Ye, F.; Wei, B.; Deng, M.; Li, T.; Huang, P.; Wang, P. J. Am. Chem. Soc. 2024, 146, 2615.
|
| [22] |
(a) Ye, F.; Li, C.; Liu, F.-L.; Liu, X.; Xu, P.; Luo, R.-H.; Song, W.; Zheng, Y.-T.; Ying, T.; Yu, B.; Wang, P. Natl. Sci. Rev. 2024, 11, nwae030.
|
|
(b) Ye, F.; Zhao, J.; Xu, P.; Liu, X.; Yu, J.; Shangguan, W.; Liu, J.; Luo, X.; Li, C.; Ying, T.; Wang, J.; Yu, B.; Wang, P. Angew. Chem., Int. Ed. 2021, 60, 12904.
|
|
| [23] |
(a) Merrifield, R. B. J. Am. Chem. Soc. 1963, 85, 2149.
|
|
(b) Carpino, L. A.; Han, G. Y. J. Am. Chem. Soc. 1970, 92, 5748.
|
|
| [24] |
Dawson, P. E.; Muir, T. W.; Clark-Lewis, I.; Kent, S. B. H. Science 1994, 266, 776.
|
| [25] |
(a) Wan, Q.; Danishefsky, S. J. Angew. Chem. Int. Ed. 2007, 46, 9248.
|
|
(b) Zhang, J.; Liu, H.; Teng, S.; Liao, Z.; Meng, L.; Wan, Q.; Dong, S. Chem. Commun. 2023, 59, 6513.
|
|
| [26] |
Maity, S. K.; Jbara, M.; Laps, S.; Brik, A. Angew. Chem., Int. Ed. 2016, 55, 8108.
|
| [27] |
(a) Fang, G.-M.; Li, Y.-M.; Shen, F.; Huang, Y.-C.; Li, J.-B.; Lin, Y.; Cui, H.-K.; Liu, L. Angew. Chem., Int. Ed. 2011, 50, 7645.
|
|
(b) Huang, Y.-C.; Fang, G.-M.; Liu, L. Natl. Sci. Rev. 2016, 3, 107.
|
|
| [28] |
Li, H.; Dong, S. Sci. China: Chem. 2017, 60, 201.
|
| [29] |
Schwarz, J. B.; Kuduk, S. D.; Chen, X.-T.; Sames, D.; Glunz, P. W.; Danishefsky, S. J. J. Am. Chem. Soc. 1999, 121, 2662.
|
| [30] |
Flood, D. T.; Hintzen, J. C. J.; Bird, M. J.; Cistrone, P. A.; Chen, J. S.; Dawson, P. E. Angew. Chem., Int. Ed. 2018, 57, 11634.
|
| [31] |
Jbara M.; Laps S.; Morgan M.; Kamnesky, G.; Mann, G.; Wolberger, C.; Brik, A. Nat. Commun. 2018, 9, 3154.
|
| [32] |
Murakami, M.; Okamoto, R.; Izumi, M.; Kajihara, Y. Angew. Chem. Int., Ed. 2012, 51, 3567.
|
| [33] |
Thompson, R. E.; Chan, B.; Radom, L.; Jolliffe, K. A.; Payne, R. J. Angew. Chem., Int. Ed. 2013, 52, 9723.
|
| [1] | Minyuan Zhou, Jie Zhao, Farong Ye, Ping Huang, Minggang Deng, Ping Wang. Semi-synthesis of Glycosylated Interferon-γ [J]. Chinese Journal of Organic Chemistry, 2024, 44(7): 2296-2304. |
| [2] | Haoruo Shangguan, Ping Huang, Zhenya Dai, Ping Wang. Synthesis and Application of β-Thiolated Amino Acids [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3089-3097. |
| [3] | Chen Chenchen, Li Sijian, Chen Yiqun, Xu Huajian, Li Yiming. Synthesis of Nesiritide via Ligation of Peptide Hydrazide [J]. Chin. J. Org. Chem., 2014, 34(7): 1452-1457. |
| [4] | Si Yanyan, Guo Ye, Li Haiyan, Sun Haoyuan, Fang Gemin. Recent Advances in Chemical Synthesis of Backbone Cyclized Peptides [J]. Chin. J. Org. Chem., 2014, 34(3): 450-460. |
| [5] | LU Jin-Rong, XING Hui, LONG Wen-Yan, LI Yun-Man, YANG Yu, HUANG Wen-Long. Synthesis, Pharmacological Activity of A Series of Genistein Deriva-tives as Modulators of Multidrug Resistance [J]. Chin. J. Org. Chem., 2011, 31(11): 1884-1892. |
| [6] | Zhang Jian;Zhang Qisheng;Tian Gengyuan. Recent Develpoment in Synthesis of Neoglycoprotein [J]. Chin. J. Org. Chem., 2003, 23(5): 425-431. |
| [7] | Zhang Hong;Guo Zongru. Synthesis and activity of glycoprotein IIb/IIIa antagonist [J]. Chin. J. Org. Chem., 2002, 22(10): 754-760. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||