Chinese Journal of Organic Chemistry ›› 2025, Vol. 45 ›› Issue (9): 3175-3185.DOI: 10.6023/cjoc202506006 Previous Articles Next Articles
REVIEWS
赵友学†, 李兮若†, 孟洛冰, 李春秀, 范贵生, 许建和*( )
)
收稿日期:2025-06-03
修回日期:2025-08-21
发布日期:2025-09-11
作者简介:† 共同第一作者
基金资助:
Youxue Zhao, Xiruo Li, Luobing Meng, Chunxiu Li, Guisheng Fan, Jianhe Xu*( )
)
Received:2025-06-03
Revised:2025-08-21
Published:2025-09-11
Contact:
E-mail: About author:† The authors contributed equally to this work.
★ Academic Papers of the 27th Annual Meeting of the China Association for Science and Technology.
Supported by:Share
Youxue Zhao, Xiruo Li, Luobing Meng, Chunxiu Li, Guisheng Fan, Jianhe Xu. Advances in Understanding the Substrate Promiscuity of Alcohol Dehydrogenases/Carbonyl Reductases★[J]. Chinese Journal of Organic Chemistry, 2025, 45(9): 3175-3185.






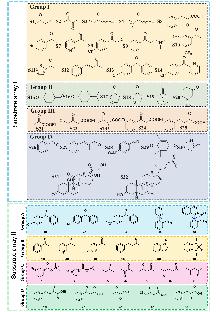



| [1] |
|
|
(江杏杏, 武卫龙, 任慧莹, 张凤, 莫文枝, 卢志强, 化学学报, 2024, 82, 736.)
|
|
| [2] |
|
| [3] |
|
|
(张文鹤, 祝天慧, 秦凤玉, 游松, 生物产业技术, 2019, 3, 54.)
|
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
doi: 10.1007/s00018-008-8588-y pmid: 19011750 |
| [9] |
doi: 10.1039/d3ob01447a pmid: 38050738 |
| [10] |
doi: 10.1021/bi00018a001 pmid: 7742302 |
| [11] |
|
|
(张晓健, 刘倩, 柳志强, 郑裕国, 化工进展, 2021, 40, 1142.)
|
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [1] | Yu Youpei, Duan Wengui, Lin Guishan, Kang Guoqiang, Wang Xiaoyu, Lei Fuhou. Synthesis, Biological Activity and Three-Dimensional Quantitative Structure-Activity Relationship (3D-QSAR) Study of Novel 4-Methyl-1,2,4-triazole-thioethers Containing gem-Dimethylcyclopropane Ring [J]. Chinese Journal of Organic Chemistry, 2020, 40(6): 1647-1657. |
| [2] | Xu Han, Zhan Mingzhe, Liu Bin, Yang Huazheng. Synthesis and Herbicidal Activity of 2-Substituted-6-methyl-4-phenyl-3(2H)-pyridazinone [J]. Chin. J. Org. Chem., 2014, 34(4): 722-728. |
| [3] | ZHU You-Quan, ZHU Ran, YUAN Yan-Wei, ZHANG Jin, WANG Wen-Hu, ZOU Xiao-Mao, HU Fang-Zhong, LIU Xiang-Ming, YANG Hua-Zheng. Synthesis and Bioactivities of Novel 3-[α-Hydroxy(indol-3-yl)- methylene]-pyrrolidine-2,4-dione Derivatives [J]. Chin. J. Org. Chem., 2010, 30(08): 1207-1211. |
| [4] |
Zhu, Youquan ; Liu, Weimin; Liu, Bin ; Hu, Fangzhong; Zhu, Ran; Zou, Xiaomao* ; Yang, Huazheng* . Design, Synthesis and Quantitative Structure-Activity Relationship Study of Herbicidal Analogues of 3-(Pyrimidin-2-yl)-2,3- dihydro-1,3,4-oxadiazol-2-ones [J]. Chin. J. Org. Chem., 2009, 29(04): 638-642. |
| [5] |
ZHU You-Quan, CHENG Jia, ZOU Xiao-Mao*, HU Fang-Zhong XIAO Yu-Hong, YANG Hua-Zheng*. Design, Synthesis and Quantitative Structure-Activity Relationship Study of Herbicidal Analogues of Pyrazolotetrazinones [J]. Chin. J. Org. Chem., 2008, 28(06): 1044-1049. |
| [6] |
ZHU You-Quan, ZOU Xiao-Mao*, LI Gong-Chun, YAO Chang-Sheng SI Xue-Kai, YANG Hua-Zheng*. Synthesis and Herbicidal Evaluation of 3-Substituted Benzyl-6- (trifluoromethyl)pyrimidine-2,4(1H,3H)-dione Deriva-tives [J]. Chin. J. Org. Chem., 2007, 27(06): 753-757. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||