Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (5): 2019-2028.DOI: 10.6023/cjoc202011038 Previous Articles Next Articles
ARTICLES
吴赛1, 陶吴晞1, 王果1, 赵斌1,*( ), 陈华杰1,*(
), 陈华杰1,*( )
)
收稿日期:2020-11-30
修回日期:2021-01-05
发布日期:2021-02-07
通讯作者:
赵斌, 陈华杰
基金资助:
Sai Wu1, Wuxi Tao1, Guo Wang1, Bin Zhao1,*( ), Huajie Chen1,*(
), Huajie Chen1,*( )
)
Received:2020-11-30
Revised:2021-01-05
Published:2021-02-07
Contact:
Bin Zhao, Huajie Chen
About author:Supported by:Share
Sai Wu, Wuxi Tao, Guo Wang, Bin Zhao, Huajie Chen. Synthesis and Optoelectronic Properties of A-D-A Type Small Molecule Acceptors Containing Isatin-Fused Acenaphthenequinone Imide Terminal Groups[J]. Chinese Journal of Organic Chemistry, 2021, 41(5): 2019-2028.


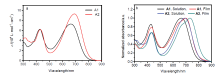
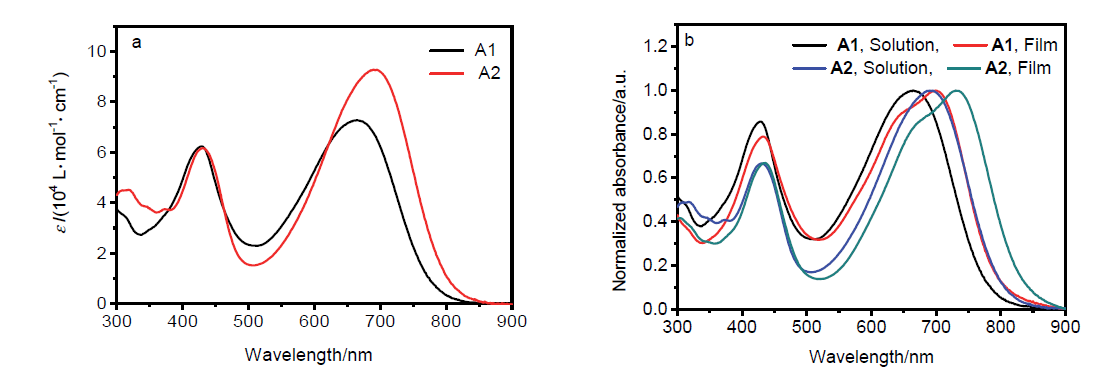
| Compd. | λmax/nm | λmaxonset/nm | Ega/eV | ELUMOb/eV | EHOMOc/eV | Egd/eV | ELUMOe/eV | EHOMOe/eV | |
|---|---|---|---|---|---|---|---|---|---|
| Solution | Film | ||||||||
| A1 | 428, 665 | 433, 700 | 825 | 1.50 | –3.94 | –5.32 | 1.42 | –3.21 | –5.08 |
| A2 | 430, 692 | 436, 733 | 858 | 1.45 | –3.97 | –5.34 | 1.37 | –3.29 | –5.09 |
| Compd. | λmax/nm | λmaxonset/nm | Ega/eV | ELUMOb/eV | EHOMOc/eV | Egd/eV | ELUMOe/eV | EHOMOe/eV | |
|---|---|---|---|---|---|---|---|---|---|
| Solution | Film | ||||||||
| A1 | 428, 665 | 433, 700 | 825 | 1.50 | –3.94 | –5.32 | 1.42 | –3.21 | –5.08 |
| A2 | 430, 692 | 436, 733 | 858 | 1.45 | –3.97 | –5.34 | 1.37 | –3.29 | –5.09 |
| 共混膜 | VOC/V | JSC/(mA?cm–2) | FF/% | PCE/% | μh/(cm2?V–1?s–1) | μe/(cm2?V–1?s–1) | μh/μe |
|---|---|---|---|---|---|---|---|
| PBDB-T:A1 | 0.96 | 8.94 | 59.22 | 5.19 | 2.20×10-4 | 5.92×10-5 | 3.72 |
| PBDB-T:A2 | 0.89 | 11.09 | 62.73 | 6.19 | 2.52×10-4 | 2.29×10-4 | 1.10 |
| 共混膜 | VOC/V | JSC/(mA?cm–2) | FF/% | PCE/% | μh/(cm2?V–1?s–1) | μe/(cm2?V–1?s–1) | μh/μe |
|---|---|---|---|---|---|---|---|
| PBDB-T:A1 | 0.96 | 8.94 | 59.22 | 5.19 | 2.20×10-4 | 5.92×10-5 | 3.72 |
| PBDB-T:A2 | 0.89 | 11.09 | 62.73 | 6.19 | 2.52×10-4 | 2.29×10-4 | 1.10 |
| [1] |
Yu, G.; Gao, J.; Hummelen, J. C.; Wudl, F.; Heeger, A. J. Science 1995, 270, 1789.
doi: 10.1126/science.270.5243.1789 |
| [2] |
Lu, L. Y.; Zheng, T. Y.; Wu, Q. H.; Schneider, A. M.; Zhao, D. L.; Yu, L. P. Chem. Rev. 2015, 115, 12666.
doi: 10.1021/acs.chemrev.5b00098 |
| [3] |
Zhang, F. L.; Inganäs, O.; Zhou, Y. H.; Vandewal, K. Natl. Sci. Rev. 2016, 3, 222.
doi: 10.1093/nsr/nww020 |
| [4] |
Sariciftci, N. S.; Smilowitz, L.; Heeger, A. J.; Wudl, F. Science 1992, 258, 1474.
doi: 10.1126/science.258.5087.1474 |
| [5] |
Liu, T.; Troisi, A. Adv. Mater. 2013, 25, 1038.
doi: 10.1002/adma.v25.7 |
| [6] |
Ganesamoorthy, R.; Sathiyan, G.; Sakthivel, P. Sol. Energy Mater Sol. Cells 2017, 161, 102.
doi: 10.1016/j.solmat.2016.11.024 |
| [7] |
Fraga Domínguez, I.; Distler, A.; Lüer, L. Adv. Energy Mater. 2017, 7, 1601320.
doi: 10.1002/aenm.201601320 |
| [8] |
Zhang, G. Y.; Zhao, J. B.; Chow, P. C. Y.; Jiang, K.; Zhang, J. Q.; Zhu, Z. L.; Zhang, J; Huang, H.; Yan, H. Chem. Rev. 2018, 118, 3447.
doi: 10.1021/acs.chemrev.7b00535 |
| [9] |
Wang, T.; Sun, R.; Shi, M. M.; Pan, F.; Hu, Z. C.; Huang, F.; Li, Y. F.; Min, J. Adv. Energy Mater. 2020, 10, 2000590.
doi: 10.1002/aenm.v10.22 |
| [10] |
Liu, Q. S.; Jiang, Y. F.; Jin, K.; Qin, J. Q.; Xu, J. G.; Li, W. T.; Xiong, J.; Liu, J. F.; Xiao, Z.; Sun, K.; Yang, S. F.; Zhang, X. T.; Ding, L. M. Sci. Bull. 2020, 65, 272.
doi: 10.1016/j.scib.2020.01.001 |
| [11] |
Li, D. Q.; Zhu, L.; Liu, X. J.; Xiao, W.; Yang, J. M.; Ma, R. R.; Ding, L. M.; Liu, F.; Duan, C. G.; Fahlman, M.; Bao, Q. Y. Adv. Mater. 2020, 32, 2002344.
doi: 10.1002/adma.v32.34 |
| [12] |
He, D.; Zhao, F. W.; Jiang, L.; Wang, C. R. J. Mater. Chem. A 2018, 6, 8839.
doi: 10.1039/C8TA02534G |
| [13] |
Wan, X. J.; Li, C. X.; Zhang, M. T.; Chen, Y. S. Chem. Soc. Rev. 2020, 49, 2828.
doi: 10.1039/D0CS00084A |
| [14] |
Yao, H. T.; Ma, L. K.; Yu, H.; Yu, J. W.; Chow, P. C. Y.; Xue, W. Y.; Zou, X. H.; Chen, Y. Z.; Liang, J. E.; Arunagiri, L.; Gao, F.; Sun, H. L.; Zhang, G.; Ma, W.; Yan, H. Adv. Energy Mater. 2020, 10, 2001408.
doi: 10.1002/aenm.v10.35 |
| [15] |
Jia, B. Y.; Wang, J.; Wu, Y.; Zhang, M. Y.; Jiang, Y. F.; Tang, Z.; Russell, T. P.; Zhan, X. W. J. Am. Chem. Soc. 2019, 141, 19023.
doi: 10.1021/jacs.9b08988 |
| [16] |
Yan, C. Q.; Wang, W.; Lau, T.-K.; Li, K. J.; Wang, J. Y.; Liu, K.; Lu, X. H.; Zhan, X. W. J. Mater. Chem. A 2018, 6, 16638.
doi: 10.1039/C8TA05800H |
| [17] |
Ye, L. L.; Xie, Y. P.; Xiao, Y. Q.; Song, J. L.; Li, C.; Fu, H. T.; Weng, K. K.; Lu, X. H.; Tan, S. T.; Sun, Y. M. J. Mater. Chem. A 2019, 7, 8055.
doi: 10.1039/C9TA01285K |
| [18] |
Lin, Y. Z.; Zhao, F. W.; He, Q.; Huo, L. J.; Wu, Y.; Parker, T. C.; Ma, W.; Sun, Y. M.; Wang, C. R.; Zhu, D. B.; Heeger, A. J.; Marder, S. R.; Zhan, X. W. J. Am. Chem. Soc. 2016, 138, 4955.
doi: 10.1021/jacs.6b02004 |
| [19] |
Yang, Y. K.; Zhang, Z. G.; Bin, H. J.; Chen, S. S.; Gao, L.; Xue, L. W.; Yang, C. D.; Li, Y. F. J. Am. Chem. Soc. 2016, 138, 15011.
doi: 10.1021/jacs.6b09110 |
| [20] |
Deng, Y. H.; Peng, A. D.; Wu, X. X.; Chen, H. J.; Huang, H. Acta Phys.-Chim. Sin. 2019, 35, 461 (in Chinses).
doi: 10.3866/PKU.WHXB201806073 |
|
(邓祎华, 彭爱东, 吴筱曦, 陈华杰, 黄辉, 物理化学学报, 2019, 35, 461.)
|
|
| [21] |
Lan, L. Y.; Chen, Z. M.; Ying, L.; Huang, F.; Cao, Y. Org. Electron. 2016, 30, 176.
doi: 10.1016/j.orgel.2015.12.022 |
| [22] |
Li, X. L.; Guo, J.; Yang, L. F.; Chao, M. H.; Zheng, L.P; Ma, Z. Y.; Hu, Y. Y.; Zhao, Y.; Chen, H. J.; Liu, Y. Q. Front. Chem. 2019, 7, 362.
doi: 10.3389/fchem.2019.00362 |
| [23] |
Li, H. Y.; Kim, F. S.; Ren, G. Q. Angew. Chem., nt. Edit. 2013, 52, 5513.
|
| [24] |
Li, H. Y.; Kim, S.; Ren, G. Q.; Jenekhe, S. A. J. Am. Chem. Soc. 2013, 135, 14920.
doi: 10.1021/ja407471b |
| [25] |
Li, H. Y.; Earmme, T.; Ren, G. Q.; Saeki, A.; Yoshikawa, S.; Murari, N. M.; Subramaniyan, S.; Crane, M. J.; Seki, S.; Jenekhe, S. A. J. Am. Chem. Soc. 2014, 136, 14589.
doi: 10.1021/ja508472j |
| [26] |
Hwang, Y. J.; Li, H. Y.; Courtright, B. A. E.; Subramaniyan, S.; Jenekhe, S. A. Adv. Mater. 2016, 28, 124.
doi: 10.1002/adma.201503801 |
| [27] |
Tan, D.; Wu, S.; Wei, H.; Hu, Y. Y.; Chen, H. J. Chin. J. Org. Chem. 2020, 40, 2919 (in Chinses)
doi: 10.6023/cjoc202005035 |
|
(谭丹, 吴赛, 魏欢, 胡袁源, 陈华杰, 有机化学, 2020, 40, 2919.)
doi: 10.6023/cjoc202005035 |
|
| [28] |
Zhao, D.; Hu, J.Y; Liu, Z.J; Xiao, B.; Wang, X. Z.; Zhou, E. J.; Zhang, Q. Dyes Pigm. 2018, 151, 102.
doi: 10.1016/j.dyepig.2017.12.054 |
| [29] |
Herrera, H.; de Echegaray, P.; Urdanpilleta, M.; Mancheno, M. J.; Mena-Osteritz, E.; Bauerle, P.; Segura, J. L. Chem. Commun. 2013, 49, 713.
doi: 10.1039/C2CC36791B |
| [30] |
Chen, H. J.; Cai, G. S.; Guo, A. K.; Zhao, Z. Y.; Kuang, J. H.; Zheng, L. P.; Zhao, L. L.; Chen, J. Y.; Guo, Y. L.; Liu, Y. Q. Macromolecules 2019, 52, 6149.
doi: 10.1021/acs.macromol.9b00834 |
| [31] |
Li, X. L.; Wu, K.L; Zheng, L. P.; Deng, Y. H.; Tan, S. T.; Chen, H. J. Dyes Pigm. 2019, 168, 59.
doi: 10.1016/j.dyepig.2019.04.027 |
| [32] |
Xin, H. S.; Ge, C. W.; Fu, L. N.; Yang, X. D.; Gao, X. K. Chin. J. Org. Chem. 2017, 37, 711 (in Chinses).
doi: 10.6023/cjoc201609029 |
|
(辛涵申, 葛从伍, 傅丽娜, 杨笑迪, 高希珂, 有机化学, 2017, 37, 711.)
doi: 10.6023/cjoc201609029 |
|
| [33] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H. Vreven, T.; Montgomery, J. A.; Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, P.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision A.02, Gaussian, Inc, Wallingford CT, 2009.
|
| [34] |
Zhang, G. B.; Zhao, Y.; Kang, B.; Park, S.; Ruan, J. F.; Lu, H. B.; Qiu, L. Z.; Ding, Y. S.; Cho, K. Chem. Mater. 2019, 31, 2027.
doi: 10.1021/acs.chemmater.8b05054 |
| [35] |
Cao, Q. F.; Xiong, W. T.; Chen, H. J.; Cai, G. S.; Wang, G.; Zheng, L. P.; Sun, Y. M. J. Mater. Chem. A 2017, 5, 7451.
doi: 10.1039/C7TA01143A |
| [36] |
Zhou, Y. X.; Xue, B.; Wu, C. Y.; Chen, S. Q.; Liu, H.; Jiu, T. G.; Li, Z. B.; Zhao, Y. J. Chem. Commun. 2019, 55, 13570.
doi: 10.1039/C9CC07040K |
| [37] |
Gao, H. L.; Yang, X. D.; Xin, H. S.; Gao, T. Z.; Gong, H. G.; Gao, X. K. Chin. J. Org. Chem. 2018, 38, 2680 (in Chinses)
doi: 10.6023/cjoc201805004 |
|
(高洪磊, 杨笑迪, 辛涵申, 高铁阵, 龚和贵, 高希珂, 有机化学, 2018, 38, 2680.)
doi: 10.6023/cjoc201805004 |
|
| [38] |
Hummelen, J. C.; Knight, B. W.; Lepeq, F.; Wudl, F.; Yao, J.; Wilkins, C. L. J. Org. Chem. 1995, 60, 532.
doi: 10.1021/jo00108a012 |
| [39] |
Cai, Y. H.; Zhang, H. T.; Ye, L. L.; Zhang, R.; Xu, J. Q.; Zhang, K. N.; Bi, P. Q.; Li, T. F.; Weng, K. K.; Xu, K.; Xia, J. L.; Bao, Q. Y.; Liu, F.; Hao, X. T.; Tan, S. T.; Gao, F.; Zhan, X. W.; Sun, Y. ACS Appl. Mater. Interfaces 2020, 12, 43984.
doi: 10.1021/acsami.0c13085 |
| [40] |
Zhou, Z. J.; Duan, J. M.; Ye, L. L.; Wang, G.; Zhao, B.; Tan, S. T.; Shen, P.; Ryu, H. S.; Woo, H. Y.; Sun, Y. M. J. Mater. Chem. A 2020, 8, 9684.
doi: 10.1039/D0TA00451K |
| [1] | Tan Dan, Wu Sai, Wei Huan, Hu Yuanyuan, Chen Huajie. Design, Synthesis, and Properties of Conjuated Molecules with Isatin-Fused Acenaphthenequinone Imide Moieties [J]. Chinese Journal of Organic Chemistry, 2020, 40(9): 2919-2928. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||