Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (6): 2485-2495.DOI: 10.6023/cjoc202011023 Previous Articles Next Articles
ARTICLES
李成飞, 岑波*( ), 段文贵*(
), 段文贵*( ), 林桂汕, 王秀, 李宝谕
), 林桂汕, 王秀, 李宝谕
收稿日期:2020-11-17
修回日期:2020-12-26
发布日期:2021-02-22
通讯作者:
岑波, 段文贵
基金资助:
Chengfei Li, Bo Cen( ), Wengui Duan(
), Wengui Duan( ), Guishan Lin, Xiu Wang, Baoyu Li
), Guishan Lin, Xiu Wang, Baoyu Li
Received:2020-11-17
Revised:2020-12-26
Published:2021-02-22
Contact:
Bo Cen, Wengui Duan
Supported by:Share
Chengfei Li, Bo Cen, Wengui Duan, Guishan Lin, Xiu Wang, Baoyu Li. Synthesis, Herbicidal Activity and Three-Dimensional Quantitative Structure-Activity Relationship (3D-QSAR) Study of 4-Methyl- 1,2,4-triazole-thioether Compounds Containing Natural Styrene Structure[J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2485-2495.
| Compd. | B. campestris | E. crusgalli | |||
|---|---|---|---|---|---|
| 10 μg/mL | 100 μg/mL | 10 μg/mL | 100 μg/mL | ||
| Flumioxazin | 57.8 | 63.0 | 95.1 | 97.5 | |
| 5a | 30.7 | 74.9 | 5.0 | 20.0 | |
| 5b | 68.9 | 86.9 | 15.0 | 40.0 | |
| 5c | 79.3 | 89.4 | 25.0 | 40.0 | |
| 5d | 41.7 | 90.5 | 0 | 0 | |
| 5e | 67.5 | 83.2 | 0 | 0 | |
| 5f | 38.3 | 76.8 | 0 | 0 | |
| 5g | 22.6 | 75.7 | 10.0 | 40.0 | |
| 5h | 25.3 | 77.4 | 35.0 | 70.0 | |
| 5i | 38.9 | 81.4 | 15.0 | 75.0 | |
| 5j | 10.0 | 47.9 | 20.0 | 50.0 | |
| 5k | 0 | 79.3 | 20.0 | 40.0 | |
| 5l | 34.0 | 73.0 | 35.0 | 55.0 | |
| 5m | 13.8 | 65.1 | 15.0 | 30.0 | |
| 5n | 4.6 | 31.8 | 10.0 | 25.0 | |
| 5o | 25.8 | 60.5 | 10.0 | 25.0 | |
| 5p | 4.6 | 18.7 | 0 | 0 | |
| 5q | 20.9 | 81.7 | 15.0 | 20.0 | |
| 5r | 29.6 | 52.3 | 20.0 | 50.0 | |
| 5s | 0 | 9.0 | 0 | 10.0 | |
| 5t | 23.7 | 57.8 | 0 | 0 | |
| 5u | 21.1 | 25.2 | 0 | 10.0 | |
| 5v | 37.2 | 79.1 | 0 | 0 | |
| 5w | 24.5 | 55.1 | 0 | 10.0 | |
| 5x | 60.7 | 83.2 | 0 | 10.0 | |
| 5y | 45.0 | 60.2 | 0 | 0 | |
| 5z | 30.8 | 85.6 | 0 | 0 | |
| Compd. | B. campestris | E. crusgalli | |||
|---|---|---|---|---|---|
| 10 μg/mL | 100 μg/mL | 10 μg/mL | 100 μg/mL | ||
| Flumioxazin | 57.8 | 63.0 | 95.1 | 97.5 | |
| 5a | 30.7 | 74.9 | 5.0 | 20.0 | |
| 5b | 68.9 | 86.9 | 15.0 | 40.0 | |
| 5c | 79.3 | 89.4 | 25.0 | 40.0 | |
| 5d | 41.7 | 90.5 | 0 | 0 | |
| 5e | 67.5 | 83.2 | 0 | 0 | |
| 5f | 38.3 | 76.8 | 0 | 0 | |
| 5g | 22.6 | 75.7 | 10.0 | 40.0 | |
| 5h | 25.3 | 77.4 | 35.0 | 70.0 | |
| 5i | 38.9 | 81.4 | 15.0 | 75.0 | |
| 5j | 10.0 | 47.9 | 20.0 | 50.0 | |
| 5k | 0 | 79.3 | 20.0 | 40.0 | |
| 5l | 34.0 | 73.0 | 35.0 | 55.0 | |
| 5m | 13.8 | 65.1 | 15.0 | 30.0 | |
| 5n | 4.6 | 31.8 | 10.0 | 25.0 | |
| 5o | 25.8 | 60.5 | 10.0 | 25.0 | |
| 5p | 4.6 | 18.7 | 0 | 0 | |
| 5q | 20.9 | 81.7 | 15.0 | 20.0 | |
| 5r | 29.6 | 52.3 | 20.0 | 50.0 | |
| 5s | 0 | 9.0 | 0 | 10.0 | |
| 5t | 23.7 | 57.8 | 0 | 0 | |
| 5u | 21.1 | 25.2 | 0 | 10.0 | |
| 5v | 37.2 | 79.1 | 0 | 0 | |
| 5w | 24.5 | 55.1 | 0 | 10.0 | |
| 5x | 60.7 | 83.2 | 0 | 10.0 | |
| 5y | 45.0 | 60.2 | 0 | 0 | |
| 5z | 30.8 | 85.6 | 0 | 0 | |
| Compd. | ED | ED'' | Residue |
|---|---|---|---|
| 5f | –1.97 | –2.03 | 0.06 |
| 5g | –2.01 | –2.00 | –0.01 |
| 5h | –1.97 | –1.97 | 0 |
| 5i | –1.89 | –1.90 | 0.01 |
| 5j | –2.56 | –2.56 | 0 |
| 5k | –1.93 | –1.90 | –0.03 |
| 5l | –2.08 | –2.03 | –0.05 |
| 5m | –2.24 | –2.23 | –0.01 |
| 5n | –2.86 | –2.84 | –0.02 |
| 5o | –2.35 | –2.32 | –0.03 |
| 5s | –3.55 | –3.55 | 0 |
| 5t | –2.47 | –2.45 | –0.02 |
| 5u | –3.03 | –3.05 | 0.02 |
| 5v | –1.96 | –2.01 | 0.05 |
| 5w | –2.49 | –2.52 | 0.03 |
| 5p* | –3.17 | –3.06 | –0.11 |
| 5q* | –1.94 | –1.98 | 0.04 |
| 5r* | –2.55 | –2.64 | 0.09 |
| Compd. | ED | ED'' | Residue |
|---|---|---|---|
| 5f | –1.97 | –2.03 | 0.06 |
| 5g | –2.01 | –2.00 | –0.01 |
| 5h | –1.97 | –1.97 | 0 |
| 5i | –1.89 | –1.90 | 0.01 |
| 5j | –2.56 | –2.56 | 0 |
| 5k | –1.93 | –1.90 | –0.03 |
| 5l | –2.08 | –2.03 | –0.05 |
| 5m | –2.24 | –2.23 | –0.01 |
| 5n | –2.86 | –2.84 | –0.02 |
| 5o | –2.35 | –2.32 | –0.03 |
| 5s | –3.55 | –3.55 | 0 |
| 5t | –2.47 | –2.45 | –0.02 |
| 5u | –3.03 | –3.05 | 0.02 |
| 5v | –1.96 | –2.01 | 0.05 |
| 5w | –2.49 | –2.52 | 0.03 |
| 5p* | –3.17 | –3.06 | –0.11 |
| 5q* | –1.94 | –1.98 | 0.04 |
| 5r* | –2.55 | –2.64 | 0.09 |
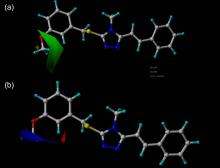
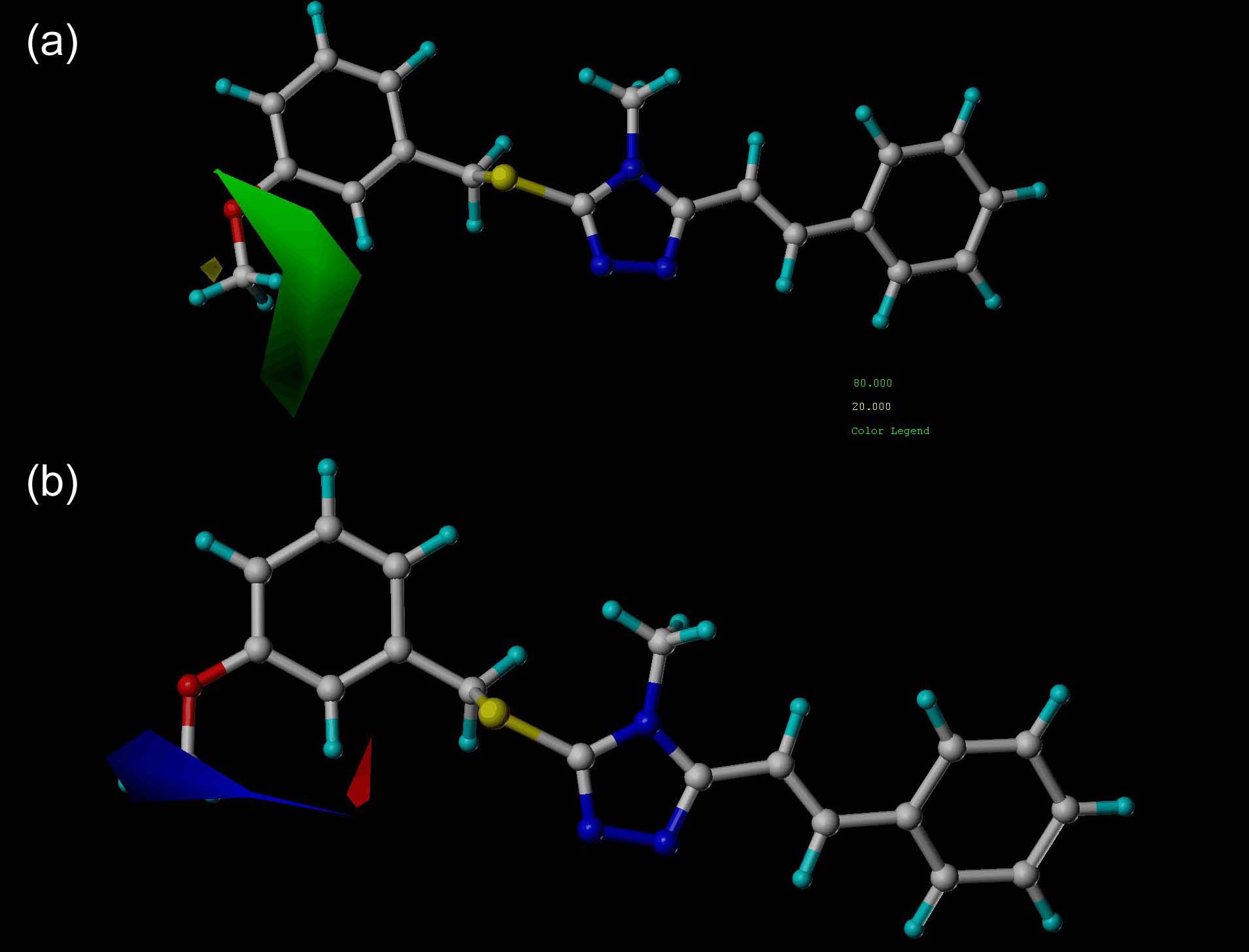


| [1] |
Yang, L.; Zhou, L. Z.; Chen, H. Y.; Gu, Y. Farm. Prod. Process 2019, 2,41(in Chinese).
|
|
( 杨漓, 周丽珠, 陈海燕, 谷瑶, 农产品加工, 2019, 2,41. )
|
|
| [2] |
Wu, C. H.; Shu, M.; Li, Q.; Ding, P. Chin. Med. J. Res. Prac. 2017, 31,14(in Chinese).
|
|
( 伍彩红, 舒眉, 李倩, 丁平, 现代中药研究与实践, 2017, 31,14.)
|
|
| [3] |
Li, Q. Z.; Song, B. A.; Cai, X. J.; Zheng, Y. G.; Guo, Q. Q. Chin. J. Org. Chem. 2010, 30,569(in Chinese).
|
|
( 李黔柱, 宋宝安, 蔡学建, 郑玉国, 郭晴晴, 有机化学, 2010, 30,569.)
|
|
| [4] |
Zhang, X. B.; Ma, H. Y.; Sun, T. D.; Lei, P.; Yang, X. L.; Zhang, X. M.; Ling, Y. Chin. J. Org. Chem. 2019, 39,2965(in Chinese).
doi: 10.6023/cjoc201903031 |
|
( 张学博, 马航宇, 孙腾达, 雷鹏, 杨新玲, 张晓鸣, 凌云, 有机化学, 2019, 39,2965.)
doi: 10.6023/cjoc201903031 |
|
| [5] |
Marona, H.; Szkaradek, N.; Karczewska, E.; Trojanowska, D.; Budak, A.; Bober, P.; Przepiorka, W.; Marek Cegla, M.; Szneler, E. Arch. Pharm. Chem. Life Sci. 2010, 342,9.
doi: 10.1002/ardp.v342:1 |
| [6] |
Xu, Y.; Lei, P.; Ling, Y.; Wang, S. W.; Yang, X. L. Chin. J. Org. Chem. 2014, 34,1118(in Chinese).
doi: 10.6023/cjoc201401034 |
|
( 徐焱, 雷鹏, 凌云, 王圣文, 杨新玲, 有机化学, 2014, 34,1118.)
doi: 10.6023/cjoc201401034 |
|
| [7] |
Friedman, M. J. Agric. Food Chem. 2017, 65,10406.
doi: 10.1021/acs.jafc.7b04344 |
| [8] |
Li, X.; sheng, J. Z.; Huang, G. H.; Ma, R. X.; Yin, F. X.; Song, D.; Zhao, C.; Ma, S. T. Eur. J. Med. Chem. 2015, 97,32.
doi: 10.1016/j.ejmech.2015.04.048 |
| [9] |
Kim, H. K.; Kim, J. R.; Ahn, Y. J. J. Stored. Prod. Res. 2004, 40,55.
doi: 10.1016/S0022-474X(02)00075-9 |
| [10] |
Saad, M. M. G.; Gouda, N. A. A.; Abdelgaleil, S. A. M. J. Environ. Sci. Heal. B 2019, 54,954.
doi: 10.1080/03601234.2019.1653121 |
| [11] |
Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Molecules 2018, 23,471.
doi: 10.3390/molecules23020471 |
| [12] |
Muñoz, M.; Torres‐Pagán, N.; Peiró, R.; Guijarro, R.; Sánchez‐Moreiras, A. M.; Verdeguer, M. Agronomy 2020, 10,791.
doi: 10.3390/agronomy10060791 |
| [13] |
Zhou, J.; Yuan, X. R.; Li, L.; Zhang, T.; Wang, B. Nat. Prod. Res. 2017, 31,2909.
doi: 10.1080/14786419.2017.1299724 |
| [14] |
Kwon, B. M.; Lee, S. H.; Choi, S. U.; Park, S. H.; lee, C. O.; Cho, Y. K.; Sung, N. D.; Bok, S. H. Arch. Pharm. Res. 1998, 21,147.
pmid: 9875422 |
| [15] |
Ka, H.; Park, H. J.; Jung, H. J.; Choi, J. W.; Cho, K. S.; Ha, J.; Lee, K. T. Cancer Lett. 2003, 196,143.
doi: 10.1016/S0304-3835(03)00238-6 |
| [16] |
Cabello, C. M.; 3rd, W. B. B.; Lamore, S. D.; Ley, S.; Bause, A. S.; Azimian, S.; Wondrak, G.T.. Free Radic. Biol. Med. 2009, 46,220.
doi: 10.1016/j.freeradbiomed.2008.10.025 |
| [17] |
Wu, W. N.; Jiang, Y. M.; Zhang, B. Y.; Wang, J. Z.; Du, H. T.; Fei, Q. Chemistry 2019, 82,1115(in Chinese).
|
|
( 吴文能, 姜阳明, 张卜艳, 王家忠, 杜海堂, 费强, 化学通报, 2019, 82,1115.)
|
|
| [18] |
He, S. C.; Zhang, H. Z.; Zhang, H. J.; Sun, Q.; Zhou, C. H. Med. Chem. 2020, 16,104.
doi: 10.2174/1573406414666181106124852 |
| [19] |
Liu, X. H.; Sun, Z. H.; Yang, M. Y.; Tan, C. X.; Weng, J. Q.; Zhang, Y. G.; Ma, Y. Chem. Biol. Drug Des. 2014, 84,342.
doi: 10.1111/cbdd.2014.84.issue-3 |
| [20] |
Rode, N. D.; Sonawane, A. D.; Nawale, L.; Khedkar, V. M.; Joshi, R. A.; Likhite, A. P.; Sarkar, D.; Joshi, R. R. Chem. Biol. Drug Des. 2017, 90,1206.
doi: 10.1111/cbdd.2017.90.issue-6 |
| [21] |
Gilandoust, M.; Harsha, K. B.; Mohan, C. D.; Raquib, A. R.; Rangappa, S.; Pandey, V.; Lobie, P. E.; Basappa
doi: S0960-894X(18)30420-7 pmid: 29789259 |
| [22] |
Korcz, M.; Saczewski, F.; Bednarski, P. J.; Kornicka, A. Molecules 2018, 23,1497.
doi: 10.3390/molecules23061497 |
| [23] |
Massari, S.; Nannetti, G.; Desantis, J.; Muratore, G.; Sabatini, S.; Manfroni, G.; Mercorelli, B.; Cecchetti, V.; Palu, G.; Cruciani, G.; Loregian, A.; Goracci, L.; Tabarrini, O. J. Med. Chem. 2015, 58,3830.
doi: 10.1021/acs.jmedchem.5b00012 pmid: 25856229 |
| [24] |
Liu, X. H.; Xu, X. Y.; Tan, C. X.; Weng, J. Q.; Xin, J. H.; Chen, J. Pest Manage. Sci. 2015, 71,292.
doi: 10.1002/ps.3804 |
| [25] |
Wang, B. L.; Zhang, L. Y.; Liu, X. H.; Ma, Y.; Zhang, Y.; Li, Z. M.; Zhang, X. Bioorg. Med. Chem. Lett. 2017, 27,5457.
doi: 10.1016/j.bmcl.2017.10.065 |
| [26] |
Wang, B. L.; Zhang, L. Y.; Zhan, Y. Z.; Zhang, Y.; Zhang, X.; Wang, L. Z.; Li, Z. M. J. Fluorine Chem. 2016, 184,36.
doi: 10.1016/j.jfluchem.2016.02.004 |
| [27] |
Xu, W. M.; Song, B. A.; Yang, S.; Hu, D. Y.; Zeng, S. Agrochemicals 2010, 49,625(in Chinese).
|
|
( 徐维明, 宋宝安, 杨松, 胡德禹, 曾松, 农药, 2010, 49,625.)
|
|
| [28] |
HRAC Classification on Mode of Action 2020. (accessed September 26, 2020). https://www.hracglobal.com.
|
| [29] |
Lu, X. L.; Zhu, X.; Zhang, M.; Wu, Q. L.; Zhou, X. D.; Li, J. K. Nat. Prod. Lett. 2019, 33,2145.
|
| [30] |
Cheng, C. R.; Zheng, Z.; Liang, R. M.; Li, F.; X. Jiang, Q. Q.; Yue, L.; Wang, Q.; Ding, J.; Liu, Y.. Chem. Nat. Compd. 2020, 56,264.
doi: 10.1007/s10600-020-03003-4 |
| [31] |
Chen, Y.; Li, P.; Chen, M.; He, J.; Su, S. J.; He, M.; Wang, H.; Xue, W. J. Heterocycl. Chem. 2020, 57,1.
doi: 10.1002/jhet.v57.1 |
| [32] |
Ruan, X. H.; Zhang, C.; Jiang, S. C.; Guo, T.; Xia, R. J.; Chen, Y.; Tang, X.; Xue, W. Molecules 2018, 23,3132.
doi: 10.3390/molecules23123132 |
| [33] |
Barrows, R. D.; Hammill, J. T.; Michael, C. Tran, M. C.; Falade, M. O.; Rice, A. L.; Davis, C. W.; Emge, T. J.; Rablen, P. R.; Guy, R. K.; Knapp, S. Bioorg. Med. Chem. 2020 , 28, 115758..
|
| [34] |
Guo, Y.; Wang, X. G.; Fan, J. P.; Zhang, Q.; Wang, Y.; Zhao, Y.; Huang, M. X.; Ding, M.; Zhang, Y. B. Roy. Soc. Open Sci. 2017, 4,171053.
|
| [35] |
Yu, Y. P.; Duan, W. G.; Lin, G. S.; Kang, G. Q.; Wang, X. Y.; Lei, F. H. Chin. J. Org. Chem. 2020, 40,1647(in Chinese).
doi: 10.6023/cjoc201912042 |
|
( 虞友培, 段文贵, 林桂汕, 康国强, 王晓宇, 雷福厚, 有机化学, 2020, 40,1647.)
doi: 10.6023/cjoc201912042 |
|
| [36] |
Li, F. Y.; Huang, L.; Zhou, X. Q.; Li, Q.; Ma, X. L.; Duan, W. G.; Wang, X. Chin. J. Org. Chem. 2020, 40,2845(in Chinese).
doi: 10.6023/cjoc202003062 |
|
( 李芳耀, 黄琳, 周小群, 李倩, 马献力, 段文贵, 王秀, 有机化学, 2020, 40,2845.)
doi: 10.6023/cjoc202003062 |
|
| [37] |
Lin, G. S.; Chen, Z. C.; Duan, W. G.; Wang, X. Y.; Lei, F. H. Chin. J. Org. Chem. 2018, 38,2085(in Chinese).
doi: 10.6023/cjoc201801043 |
|
( 林桂汕, 陈智聪, 段文贵, 王晓宇, 雷福厚, 有机化学, 2018, 38,2085.)
doi: 10.6023/cjoc201801043 |
|
| [38] |
He, Y.; Duan, W. G.; Lin, G. S.; Cen, B.; Bu, J. W.; Lei, F. H. Chem. Ind. Forest Prod. 2020, 40,76(in Chinese).
|
|
( 何云, 段文贵, 林桂汕, 岑波, 卜俊文, 雷福厚, 林产化学与工业, 2020, 40,76.)
|
|
| [39] |
Lin, G. S.; Bai, X.; Duan, W. G.; Cen, B.; Huang, M.; Lu, S. Z. ACS Sustainable Chem. Eng. 2019, 7,7862.
doi: 10.1021/acssuschemeng.9b00254 |
| [40] |
Lin, G. S.; Duan, W. G.; Yang, L. X.; Huang, M.; Lei, F. H. Molecules 2017, 22,193.
doi: 10.3390/molecules22020193 |
| [41] |
Verma, J.; Khedkar, V. M.; Coutinho, E. C. Curr. Top. Med. Chem. 2010, 10,95.
doi: 10.2174/156802610790232260 |
| [42] |
Li, L. H.; Li, Z. R.; Liu, M. L.; Shen, W. Y.; Wang, B.; Guo, H. Y.; Lu, Y. Molecules 2015, 21,49.
doi: 10.3390/molecules21010049 |
| [43] |
Liu, M.X; Li, C. J.. Angew. Chem. Int. Ed. 2016, 55,10806.
doi: 10.1002/anie.201604847 |
| [44] |
Li, L. H.; Li, Z. R.; Liu, M. L.; Shen, W. Y.; Wang, B.; Guo, H. Y.; Lu, Y. Molecules 2016, 21,49.
doi: 10.3390/molecules21010049 |
| [45] |
Su, N. N.; Li, Y.; Yu, S. J.; Zhang, X.; Liu, X. H.; Zhao, W. G. Res. Chem. Intermediat. 2012, 39,759.
doi: 10.1007/s11164-012-0595-9 |
| [1] | Hai Lin, Huixiang Nie, Anlin Zhao, Tao Wang, Jin Luo. Synthesis and Herbicidal Activity of Novel Pyrimido[5,4-e]-[1,2,4]triazolo[1,5-c]pyrimidine Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2462-2475. |
| [2] | Jinjing Li, Lijiao Sun, Yan Zhao, Chengyang Shi. Research Progress on Reactions Involving β-Nitrostyrene [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4168-4187. |
| [3] | Lei Wang, Shujing Yu, Na Yang, Baolei Wang. Studies on the Synthesis and Biological Activities of Novel Dihydroquinazolinone-Containing Caffeine Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 299-307. |
| [4] | Fasheng Shi, Shengwen Wang, Huan Xu, Xingxing Lu, Xinling Yang, Tengda Sun, Changkai Wang, Xiaoming Zhang, Qing Yang, Yun Ling. Design, Synthesis and Fungicidal Actiνity of Noνel Thiosemicarbazide Compounds [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2106-2116. |
| [5] | Chaochao Wang, Hui Liu, Wei Zhao, Pan Li, Lusha Ji, Renmin Liu, Kang Lei, Xiaohua Xu. Synthesis and Herbicidal Activity of 5-(1-Amino-2-phenoxyethylidene)barbituric Acid Derivatives [J]. Chinese Journal of Organic Chemistry, 2021, 41(5): 2063-2073. |
| [6] | He Shuwang, Yan Shiqiang, Guo Wei, Zhai Guangxi, Zhang Wei. One-Pot Synthesis of Amino Alcohols from Styrenes [J]. Chinese Journal of Organic Chemistry, 2020, 40(7): 2094-2098. |
| [7] | Yang Sen, Ding Chengrong, Liu Xinghai, Weng Jianquan, Yuan Jing, Tan Chengxia. Synthesis and Herbicidal Activity of Chiral Aryloxyphenoxypropionic Amides Compounds [J]. Chinese Journal of Organic Chemistry, 2019, 39(12): 3588-3593. |
| [8] | Wang Jian-Yong, Ma Lan, Li Yan, Wang Xi-Sheng. Trifluoromethylthiolation/Oxidation of Styrenes for Facile Synthesis of α-Trifluoromethylthio Acetophenons [J]. Chin. J. Org. Chem., 2019, 39(1): 232-237. |
| [9] | Zhang Juan, Wang Biyun, Liu Yisen, Cao Song. Stereoselective Synthesis of Z-Fluorostyrene Derivatives via Nickel-Catalyzed Cross-Coupling of gem-Difluorostyrenes with Organozinc Reagents [J]. Chin. J. Org. Chem., 2019, 39(1): 249-256. |
| [10] | Shi Yujun, Du Xianchao, Wang Xianglong, Chen Qingwen, Li Ling, Dai Hong, Xu Caiqin, Zhang Jingyuan, Ling Yong. Synthesis and Herbicidal Activity of Novel Cyanoacrylate Derivatives Containing Substituted Oxazole Moiety [J]. Chin. J. Org. Chem., 2018, 38(7): 1772-1778. |
| [11] | Wu Qiongyou, Zhang Rui, Pan Jinhuan, Clough John, Gu Yucheng, Yang Guangfu. Design and Synthesis of Natural Product Mevalocidin Chiral Center Based Analogues [J]. Chin. J. Org. Chem., 2018, 38(4): 840-845. |
| [12] | Qiao Lili, Wei Yan, Hao Shuanghong. Synthesis and Biological Activity of Novel Fluorinated Amide Hydroxy Methyl Coumarin Derivatives [J]. Chin. J. Org. Chem., 2018, 38(2): 509-518. |
| [13] | Chen Wei, Li Yuxin, Li Yonghong, Yu Shujing, Xiong Lixia, Li Zhengming. Synthesis and Biological Activity of 2-Cyanobenzenesulfonylurea Derivatives [J]. Chin. J. Org. Chem., 2018, 38(10): 2747-2753. |
| [14] | Ullah Aziz, Zhang Sheng, Bao Ming. Facile Synthesis of β-Methylstyrenes via Pd/TsOH-Catalyzed Isomerization of Allylbenzenes [J]. Chin. J. Org. Chem., 2017, 37(5): 1278-1283. |
| [15] | Dai Hong, Chen Jia, Hong Yu, Yuan Binying, Chen Yumeng, Shi Yujun, Ma Ruiyuan, Liang Zhipeng, Shi Jian. Synthesis and Herbicidal Activity of Novel Cyanoacrylates Containing Substituted Pyridyl Moiety [J]. Chin. J. Org. Chem., 2017, 37(3): 739-745. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||