Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (3): 819-829.DOI: 10.6023/cjoc202107064 Previous Articles Next Articles
ARTICLES
收稿日期:2021-07-30
修回日期:2021-10-12
发布日期:2021-11-17
通讯作者:
罗稳, 田智勇
基金资助:
Yongmei Zhaoa, Yeshu Mub, Wen Luob( ), Zhiyong Tianc(
), Zhiyong Tianc( )
)
Received:2021-07-30
Revised:2021-10-12
Published:2021-11-17
Contact:
Wen Luo, Zhiyong Tian
Supported by:Share
Yongmei Zhao, Yeshu Mu, Wen Luo, Zhiyong Tian. Synthesis of Naphthalimide Derivatives as Cholinesterase Inhibitors with Aggregation Induced Emission Properties[J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 819-829.
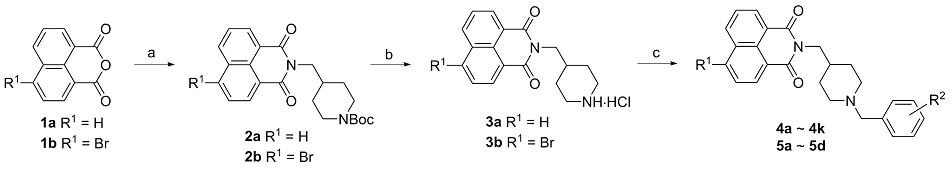
| Compd. | R1 | | IC50/(μmol•L–1) for AChEa | IC50/(μmol•L–1) for BChEb | SIc |
|---|---|---|---|---|---|
| 4a | H | | 18.43±1.96 | >100 | <0.18 |
| 4b | H | | 12.28±1.79 | >100 | <0.12 |
| 4c | H | | 9.15±0.55 | >100 | <0.09 |
| 4d | H | | 11.19±1.13 | >100 | <0.11 |
| 4e | H | | 4.66±0.54 | >100 | <0.05 |
| 4f | H | | 12.98±1.78 | >100 | <0.13 |
| 4g | H | | 8.86±1.05 | >100 | <0.09 |
| 4h | H | | 7.43±0.71 | >100 | <0.07 |
| 4i | H | | 12.72±1.92 | >100 | <0.13 |
| 4j | H | | 11.57±1.06 | >100 | <0.12 |
| 4k | H | | 4.43±0.45 | >100 | <0.04 |
| 5a | Br | | 17.14±1.69 | >100 | <0.17 |
| 5b | Br | | 14.52±0.75 | >100 | <0.15 |
| 5c | Br | | 9.58±0.81 | 41.36±2.23 | 0.23 |
| 5d | Br | | 14.30±1.30 | >100 | <0.14 |
| Riv. d | — | — | 7.25±0.34 | 1.85±0.21 | 3.92 |
| Dpz. e | — | — | 0.027±0.003 | 1.155±0.03 | 0.023 |
| Compd. | R1 | | IC50/(μmol•L–1) for AChEa | IC50/(μmol•L–1) for BChEb | SIc |
|---|---|---|---|---|---|
| 4a | H | | 18.43±1.96 | >100 | <0.18 |
| 4b | H | | 12.28±1.79 | >100 | <0.12 |
| 4c | H | | 9.15±0.55 | >100 | <0.09 |
| 4d | H | | 11.19±1.13 | >100 | <0.11 |
| 4e | H | | 4.66±0.54 | >100 | <0.05 |
| 4f | H | | 12.98±1.78 | >100 | <0.13 |
| 4g | H | | 8.86±1.05 | >100 | <0.09 |
| 4h | H | | 7.43±0.71 | >100 | <0.07 |
| 4i | H | | 12.72±1.92 | >100 | <0.13 |
| 4j | H | | 11.57±1.06 | >100 | <0.12 |
| 4k | H | | 4.43±0.45 | >100 | <0.04 |
| 5a | Br | | 17.14±1.69 | >100 | <0.17 |
| 5b | Br | | 14.52±0.75 | >100 | <0.15 |
| 5c | Br | | 9.58±0.81 | 41.36±2.23 | 0.23 |
| 5d | Br | | 14.30±1.30 | >100 | <0.14 |
| Riv. d | — | — | 7.25±0.34 | 1.85±0.21 | 3.92 |
| Dpz. e | — | — | 0.027±0.003 | 1.155±0.03 | 0.023 |

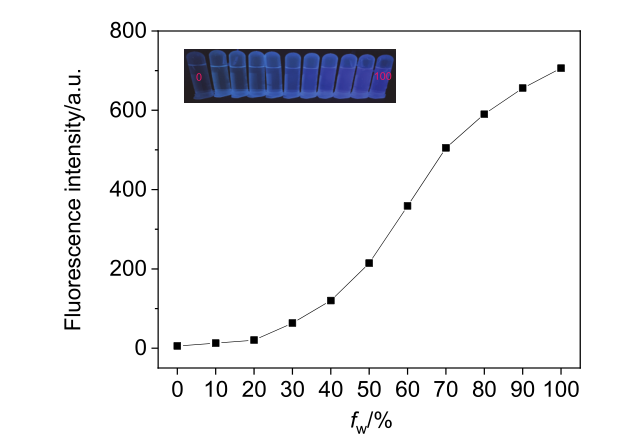


| [1] |
https://finance.sina.com.cn/china/gncj/2021-05-11/doc-ikmxzfmm1763811.shtml.
|
| [2] |
Scarpini, E.; Scheltens, P.; Feldman, H. Lancet Neurol. 2003, 2, 539.
pmid: 12941576 |
| [3] |
Mimica, N.; Presečki, P. Psychiatr. Danub. 2009, 21, 108.
|
| [4] |
Du, C.-Q.; Xie, B.-H.; He, M.; Hu, Z.-Y.; Liu, Y.; He, X.; Liu, F.-Y.; Cheng, C.; Zhou, H.-B.; Huang, S.-T.; Dong, C.-E. Chin. J. Org. Chem. 2020, 40, 2035. (in Chinese)
doi: 10.6023/cjoc202002039 |
|
(杜川黔, 谢宝花, 贺明, 胡志烨, 刘豫, 何雪, 刘凡玉, 程晨, 周海兵, 黄胜堂, 董春娥, 有机化学, 2020, 40, 2035.)
doi: 10.6023/cjoc202002039 |
|
| [5] |
Zheng, S.-Y.; Yu, C.-H.; Shen, Z.-W. Chin. Chin. J. Org. Chem. 2013, 33, 2261. (in Chinese)
doi: 10.6023/cjoc201306006 |
|
(郑书岩, 郁春辉, 沈征武, 有机化学, 2013, 33, 2261.)
doi: 10.6023/cjoc201306006 |
|
| [6] |
Bortolami, M.; Rocco, D.; Messore, A.; Di Santo, R.; Costi, R.; Madia, V. N.; Scipione, L.; Pandolfi, F. Expert Opin. Ther. Pat. 2021, 31, 399.
doi: 10.1080/13543776.2021.1874344 pmid: 33428491 |
| [7] |
Sang, Z.; Qiang, X.; Li, Y.; Xu, R.; Cao, Z.; Song, Q.; Wang, T.; Zhang, X.; Liu, H.; Tan, Z.; Deng, Y. Eur. J. Med. Chem. 2017, 135, 307.
doi: 10.1016/j.ejmech.2017.04.054 |
| [8] |
Xu, Z.; Xiao, Y.; Qian, X.; Cui, J.; Cui, D. Org. Lett. 2005, 7, 889.
doi: 10.1021/ol0473445 |
| [9] |
Duke, R. M.; Veale, E. B.; Pfeffer, F. M.; Kruger, P. E.; Gunnlaugsson, T. Chem. Soc. Rev. 2010, 39, 3936.
doi: 10.1039/b910560n |
| [10] |
Xie, Z.-D.; Fu, M.-L.; Yin, B.; Zhu, Q. Chin. J. Org. Chem. 2018, 38, 1364. (in Chinese)
doi: 10.6023/cjoc201712031 |
|
(谢振达, 付曼琳, 尹彪, 朱勍, 有机化学, 2018, 38, 1364.)
doi: 10.6023/cjoc201712031 |
|
| [11] |
Tomczyk, M. D.; Walczak, K. Z. Eur. J. Med. Chem. 2018, 159, 393.
doi: 10.1016/j.ejmech.2018.09.055 |
| [12] |
Stone, R. M.; Mazzola, E.; Neuberg, D.; Allen, S. L.; Pigneux, A.; Stuart, R. K.; Wetzler, M.; Rizzieri, D.; Erba, H. P.; Damon, L.; Jang, J. H.; Tallman, M. S.; Warzocha, K.; Masszi, T.; Sekeres, M. A.; Egyed, M.; Horst, H. A.; Selleslag, D.; Solomon, S. R.; Venugopal, P.; Lundberg, A. S.; Powell, B. J. Clin. Oncol. 2015, 33, 1252.
doi: 10.1200/JCO.2014.57.0952 |
| [13] |
Chen, Z.; Xu, Y.; Qian, X. Chin. Chem. Lett. 2018, 29, 1741.
doi: 10.1016/j.cclet.2018.09.020 |
| [14] |
Tandon, R.; Luxami, V.; Kaur, H.; Tandon, N.; Paul, K. Chem. Rec. 2017, 17, 956.
doi: 10.1002/tcr.v17.10 |
| [15] |
Rong, R.-X.; Li, J.-M.; Li, Y.-F.; Guo, X.-Y.; Wang, C.; Li, Y.-J.; Li, J.-M.; Han, B.-J.; Cao, Z.-R.; Wang, K.-R.; Li, X.-L. Chin. J. Org. Chem. 2021, 41, 1599. (in Chinese)
doi: 10.6023/cjoc202008015 |
|
(戎瑞雪, 李基民, 李耀文, 郭啸宇, 王冲, 李艳军, 李金梅, 韩宝君, 曹志然, 王克让, 李小六, 有机化学, 2021, 41, 1599.)
doi: 10.6023/cjoc202008015 |
|
| [16] |
Luo, J.; Xie, Z.; Lam, J. W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H. S.; Zhan, X.; Liu, Y.; Zhu, D.; Tang, B. Z. Chem. Commun. 2001, 18, 1740.
|
| [17] |
Gopikrishna, P.; Meher, N.; Iyer, P. K. ACS Appl. Mater. Interfaces 2018, 10, 12081.
doi: 10.1021/acsami.7b14473 |
| [18] |
Ma, Y.; Zhao, L.; Li, Y.; Liu, J.; Yang, Y.; Chu, T. Tetrahedron 2018, 74, 2684.
doi: 10.1016/j.tet.2018.04.034 |
| [19] |
Srivastava, A. K.; Singh, A. K.; Kumari, N.; Yadav, R.; Gulino, A.; Speghini, A.; Nagarajan, R.; Mishra, L. J. Lumin. 2017, 182, 274.
doi: 10.1016/j.jlumin.2016.10.042 |
| [20] |
Ma, X.; Chi, W.; Han, X.; Wang, C.; Liu, S.; Liu, X.; Yin, J. Chin. Chem. Lett. 2021, 32, 1790.
doi: 10.1016/j.cclet.2020.12.031 |
| [21] |
Gao, J.; Midde, N.; Zhu, J.; Terry, A. V.; McInnes, C.; Chapman, J. M. Bioorg. Med. Chem. Lett. 2016, 26, 5573.
doi: 10.1016/j.bmcl.2016.09.072 |
| [22] |
Blaikie, L.; Kay, G.; Kong Thoo Lin, P. Bioorg. Med. Chem. Lett. 2020, 30, 127505.
doi: 10.1016/j.bmcl.2020.127505 |
| [23] |
Gao, J.; Suo, C.; Tseng, J. H.; Moss, M. A.; Terry, A. V., Jr.; Chapman, J. Int. J. Mol. Sci. 2021, 22, 3120.
doi: 10.3390/ijms22063120 |
| [24] |
Dai, F.; Li, Q.; Wang, Y.; Ge, C.; Feng, C.; Xie, S.; He, H.; Xu, X.; Wang, C. J. Med. Chem. 2017, 60, 2071.
doi: 10.1021/acs.jmedchem.6b01846 |
| [25] |
Li, M.; Wang, Y.; Ge, C.; Chang, L.; Wang, C.; Tian, Z.; Wang, S.; Dai, F.; Zhao, L.; Xie, S. Eur. J. Med. Chem. 2018, 143, 1732.
doi: 10.1016/j.ejmech.2017.10.069 |
| [26] |
Alonso, D.; Dorronsoro, I.; Rubio, L.; Muñoz, P.; García-Palomero, E.; Monte, M. D.; Bidon-Chanal, A.; Orozco, M.; Luque, F. J.; Castro, A.; Medina, M.; Martínez, A. Bioorg. Med. Chem. 2005, 13, 6588.
doi: 10.1016/j.bmc.2005.09.029 |
| [27] |
Ellman, G. L.; Courtney, K. D.; Andres Jr, V.; Feather-Stone, R. M. Biochem. Pharmacol. 1961, 7, 88.
doi: 10.1016/0006-2952(61)90145-9 |
| [28] |
Elsinghorst, P. W.; Tanarro, C. M.; Gutschow, M. J. Med. Chem. 2006, 49, 7540.
pmid: 17149883 |
| [29] |
Mao, F.; Chen, J.; Zhou, Q.; Luo, Z.; Huang, L.; Li, X. Bioorg. Med. Chem. Lett. 2013, 23, 6737.
doi: 10.1016/j.bmcl.2013.10.034 |
| [30] |
Sukumaran, S. D.; Nasir, S. B.; Tee, J. T.; Buckle, M. J. C.; Othman, R.; Rahman, N. A.; Lee, V. S.; Bukhari, S. N. A.; Chee, C. F. J. Enzyme Inhib. Med. Chem. 2021, 36, 130.
doi: 10.1080/14756366.2020.1847100 |
| [1] | Wu Zhou, Min Peng, Qingxiang Liang, Aibin Wu, Wenming Shu, Weichu Yu. A Novel Turn-On Fluorescent Probe Based on Naphthalimide for Highly Selective and Sensitive Detection of Hydrogen Sulfide in Solution and Gas [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4277-4283. |
| [2] | Chuntian Shi, Mei Yu, Aibin Wu, Jiangxiong Luo, Xiaojun Li, Ningchen Wang, Wenming Shu, Weichu Yu. A Water-Soluble Naphthalimide-Based Fluorescent Probe for Specific Sensing of Fe3+ and $\text{C}{{\text{r}}_{2}}\text{O}_{7}^{2-}$ [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2806-2813. |
| [3] | Huixu Lu, Yonghe Tang, Hongmei Zhou, Weiying Lin. Synthesis and Study of Performance for An Enhanced Formaldehyde Fluorescent Probe [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1163-1169. |
| [4] | Yunle Lu, Yanjie Wang, Liangliang Zhu, Bingbing Yue. Progress in Synthesis and Aggregation-Induced Phosphorescence of Persulfurated Arene Compounds [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3549-3561. |
| [5] | Pengpeng Xia, Jiangtai Chen, Gaofan Shi, Mengmeng Zhang, Wanchen Yao, Xiangde Lin, Dongdong Zeng. Research Progress of Naphthalimide Derivatives Optical Probes for Monitoring Physical and Chemical Properties of Microenvironment and Active Sulfur Substances [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3620-3639. |
| [6] | Ruixue Rong, Jimin Li, Yaowen Li, Xiaoyu Guo, Chong Wang, Yanjun Li, Jinmei Li, Baojun Han, Zhiran Cao, Kerang Wang, Xiaoliu Li. Synthesis and Anti-tumor Effects of Naphthalimide Derivatives Targeted in Cell Nucleus [J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1599-1606. |
| [7] | Enqing Chen, Yonghe Tang, Lei Wang, Jiangbo Ren, Weiying Lin. Development of a New Carbon Monoxide Fluorescent Probe Based on Nitro Reduction and Its Bioimaging Research in Living Cells [J]. Chinese Journal of Organic Chemistry, 2021, 41(3): 1200-1206. |
| [8] | Xinyu Wang, Shaolong Qi, Jianshi Du, Qiang Li, Lubao Zhu, Longqi Xue, Qing Zhao, Qingbiao Yang, Yaoxian Li, Xianling Cong. A Naphthalimide-Based Hypochlorous Acid-Selective Fluorescent Probe and Its Application in Cell Imaging [J]. Chinese Journal of Organic Chemistry, 2021, 41(2): 719-725. |
| [9] | Du Chuanqian, Xie Baohua, He Ming, Hu Zhiye, Liu Yu, He Xue, Liu Fanyu, Cheng Chen, Zhou Hai-Bing, Huang Shengtang, Dong Chun'e. Design, Synthesis and Biological Evaluation of Pyrano[2,3-b]-naphthoquinone Derivatives as Acetylcholinesterase Inhibitors [J]. Chinese Journal of Organic Chemistry, 2020, 40(7): 2035-2044. |
| [10] | Yang Yujie, Xu Li, Wang Hua. Synthesis of Pyrene-Cyclooctatetrathiophene Derivatives and Their Behaviors of Photoluminescence [J]. Chinese Journal of Organic Chemistry, 2020, 40(6): 1658-1664. |
| [11] | Liu Wandong, Yang Yu, Li Jiaming, Guo Yanyan, Jin Fan, Zhang Bin. Design, Synthesis and Evaluation of Novel Tacrine-3-n-butylphthalide Hybrids as Multifunctional Cholinesterase Inhibitors [J]. Chinese Journal of Organic Chemistry, 2019, 39(12): 3505-3515. |
| [12] | Zhang Youming, Han Bingbing, Lin Qi, Mao Pengpeng, Chen Jinfa, Yao Hong, Wei Taibao. Synthesis and Fe3+ Sensing Properties of the Chemosensor Based on Functionalized Naphthalimide Schiff Base Derivative [J]. Chin. J. Org. Chem., 2018, 38(7): 1800-1805. |
| [13] | Xie Zhenda, Fu Manlin, Yin Biao, Zhu Qing. Research Progress in 1, 8-Naphthalimide-Based Fluorescent Probes for Two-Photon Imaging [J]. Chin. J. Org. Chem., 2018, 38(6): 1364-1376. |
| [14] | Zhang Weijie, Xu Li, Song Jinsheng, Ma Zhiying, Wang Hua. Synthesis of Saddle-Shaped Cyclooctatetrathiophene-Triazine Derivatives and Their Aggregation Induced Emissions (AIE) Properties [J]. Chin. J. Org. Chem., 2018, 38(5): 1119-1125. |
| [15] | Yang Suhua, Yan Sujun, Yang Jing, Zhang Ce, Han Guoying. Synthesis and Properties of Novel Dual-Color Fluorescent Molecular Switch with 1,8-Naphthalimide Unit [J]. Chin. J. Org. Chem., 2018, 38(2): 425-431. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||