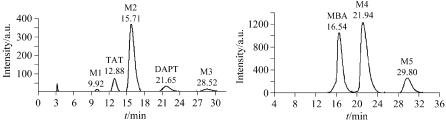
1,3,5,7-Tetraacetyl-1,3,5,7-tetraazacyclooctane (TAT) was a key intermediates for the synthesis of 1,3,5,7-tetranitro- 1,3,5,7-tetrazocine (HMX) that is a highly energetic material. TAT can be prepared from the acetylation of 3,7-diacetyl- 1,3,5,7-tetraazacyclooctane (DAPT) and the small molecules condensation reaction. In this paper, sem preparative high performance piquid chromatography (SPHPLC) was used as a new separation and purification method for the intermediates in the reaction solution, and six samples obtained with high purity (>97%) analyzed by HPLC could prove much evidence and information for the reaction mechanism research. The optimum technological parameters of SPHPLC as follows: Si column (20 mm×250 mm, 10~20 μm), V(acetonitrile)∶V(water)=95∶5 (for the method of the acetylation of DAPT), V(acetonitrile)∶ V(methanol)=90∶10 (for the method of the small molecules condensation reaction), 10 mL·min-1, 215 nm, 1 mL (30 mg· mL-1).
LI Qing-Xia
,
WANG Peng
,
LOU Zhong-Liang
,
LIU Yue
,
MENG Zi-Hui
,
XU Zhi-Bin
,
XUE Min
. Separation of Key Intermediates in the Synthesis of 1,3,5,7-Tetranitro-1,3,5,7-tetrazocine by Semi-preparative High Performance Liquid Chromatography[J]. Chinese Journal of Organic Chemistry, 2012
, 32(01)
: 165
-168
.
DOI: 10.6023/cjoc1106091
[1] Agrawal, J. P.; Hodgson, R. D. Textbook of Organic Chemistry of Explosives, John Wiley and Sons. Ltd, England, 2007, pp. 247~ 261.
[2] Cooney, A. P.; Crampton, M. R.; Jones, M.; Golding, P. J. Heterocycl. Chem. 1987, 24, 1163.
[3] Agadzhanyan, T. E.; Minasyan, G. G. Arm. Khim. Zh. 1982, 35, 315.
[4] Siele, V. I.; Warman, M.; Gilbert, E. E. J. Heterocycl. Chem. 1974, 11, 237.
[5] Lukasavage, W. J.; Behrmann, L. A.; Voreck, W. E. WO 2000073 285, 2000 [Chem. Abstr. 1983, 134, 861664].
[6] Wang, P.; Meng, Z.-H.; Li, J.-M.; Lou, Z.-L.; Xu, Z.-B. CN 101863849, 2010 [Chem. Abstr. 2010, 153, 622415].
[7] Zhang, Y.; Jiao, J.-J.; Liu, C.-M. Food Chem. 2008, 107, 1326.
[8] Nazemiyeh, H.; Rahman, M. M.; Gibbons, S. J. Nat. Med. 2008, 62, 91.
[9] Hagelin, C.; Oulie, I.; Raknes, A. J. Chromatogr. B 2004, 811, 243.
[10] Lou, Z.-L.; Meng, Z.-H.; Meng, W.-J.; Wang. P. Chin. J. Energ. Mater. 2010, 18, 226 (in Chinese). (娄忠良, 孟子晖, 孟文君, 王鹏, 含能材料, 2010, 18, 226.)
[11] Song, H.-Y.; Wang, P.; Qin, G.-M. Chin. J. Org. Chem. 2010, 30, 414 (in Chinese). (宋红燕, 王鹏, 覃光明, 有机化学, 2010, 30, 414.)
[12] Li, Q.-X.; Wang, P.; Meng, W.-J. Chin. J. Org. Chem. 2009, 29, 409 (in Chinese). (李清霞, 王鹏, 孟文君, 孟子晖, 有机化学, 2009, 30, 409.)
[13] Lou, Z.-L.; Wang, P.; Meng, Z.-H. Chin. J. Org. Chem. 2010, 30, 1059 (in Chinese). (娄忠良, 王鹏, 孟子晖, 有机化学, 2010, 30, 1059.)