

Chinese Journal of Organic Chemistry >
Bromination of 3,5-Dialkyloxytoluene
Received date: 2011-06-22
Revised date: 2011-08-24
Online published: 2011-09-19
Supported by
Project supported by the National Natural Science Foundation of China (No. 21076078).
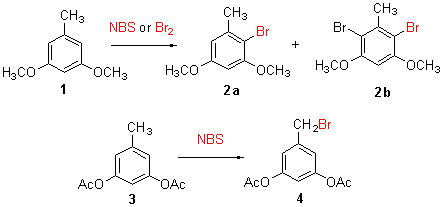
GU Ye , SHI Hong-Wei , CHEN Li-Yuan , SHEN Yong-Jia . Bromination of 3,5-Dialkyloxytoluene[J]. Chinese Journal of Organic Chemistry, 2012 , 32(01) : 174 -177 . DOI: 10.6023/cjoc1106221
/
| 〈 |
|
〉 |