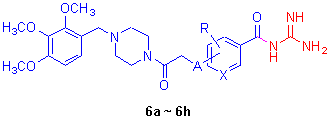
Na+/H+ exchanger 1 (NHE1) inhibitors have a protective effect against myocardial ischemic-reperfusion injury. By choseing benzoyl (or pyridine-3-carbonyl) guanidine as main structure, and introducing 4-(2,3,4-trimethoxybenzyl)piperazine- 1-yl to its benzene (or pyridine) ring, eight compounds were designed and synthesized. All the target compounds have not been reported and their structures have been confirmed by IR, 1H NMR, MS and elemental analysis. The rat platelet swelling assay (PSA) results showed that most of the tested compounds exhibited potent NHE1 inhibitory effects.
JIN Lu
,
JIANG Xiang-Min
,
HE Guang-Wei
,
XU Wen-Ting
,
GONG Guo-Qing
,
XU Yun-Gen
. Synthesis and Na+/H+ Exchanger-1-Inhibitory Activity of 4(6)-{2-[4-(2,3,4-Trimethoxybenzyl)piperazin-1-yl]-2-oxoethoxy}-aroylguanidine Derivatives[J]. Chinese Journal of Organic Chemistry, 2012
, 32(02)
: 326
-332
.
DOI: 10.6023/cjoc1108051
[1] Malo, M. E.; Fliegel, L. Can. J. Physiol. Pharmacol. 2006, 84, 1081.
[2] Masereel, B.; Pochet, L.; Laeckmann, D. Eur. J. Med. Chem. 2003, 38, 547.
[3] Karmazyn, M.; Sawyer, M.; Fliegel, L. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005, 5, 323.
[4] Park, H. S.; Lee, B. K.; Park, S.; Kim, S. U.; Lee, S. H.; Baik, E. J.; Lee, S.; Yi, K. Y.; Yoo, S. E.; Moon, C. H.; Jung, Y. S. Brain Res. 2005, 1061, 67.
[5] Zhang, R.; Lei, L.; Xu, Y. G.; Hua, W. Y.; Gong, G. Q. Bioorg. Med. Chem. Lett. 2007, 17, 2430.
[6] Jiang, X. M.; Du, P.; Xu, Y. G.; Zhang, D.; Gong, G. Q.; You, Q. D. Chin. J. Org. Chem. 2008, 28(12), 2142 (in Chinese). (姜向敏, 杜萍, 徐云根, 张迪, 尤启冬, 有机化学, 2008, 28(12), 2142.)
[7] Xu, W. T.; Jin, N.; Xu, J.; Xu, Y. G.; Wang, Q. J.; You, Q. D. Bioorg. Med. Chem. Lett. 2009, 19, 3283.
[8] Jin, L.; Jiang, X. M.; Du, P.; Xu, J.; Gong, G. Q.; Wang, Q. J.; Xu, Y. G. Eur. J. Med. Chem. 2011, 46, 4107.
[9] Rosskopf, D.; Morgenstern, E.; Scholz, W.; Osswald, U.; Siffert, W. J. Hypertens 1991, 9, 231.