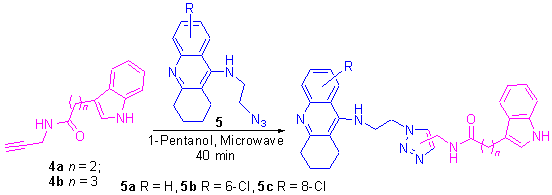
3-(Propargyl-propionamine)indole (4a) and 3-(propargyl-butyramide)indole (4b) were prepared from indole-3-propionic acid (3a) and indole-3-butyric acid (3b) by condensation with propargylamine, respectively. Twelve novel acetylcholinesterase inhibitors, tacrine-indole hybrids, were synthesized from 4a, 4b and 9-(azido-ethyl-amino)- 1,2,3,4-tetrahydroacridine derivatives (5a~5c) by microwave-assisted Huisgen [3+2] cycloaddition reaction. These novel compounds were determined by NMR, IR and HRMS techniques. The preliminary bioassay showed that all of them exhibited better AChE (electrophorus) inhibitory activity. Particularly, the IC50 values of compounds 2b and 2d were 1.6 and 2.0 nmol· L-1 respectively, which were 6.9 and 5.5 times respectively more potent than N-[6-(6-chloro-1,2,3,4-tetrahydroacridin-9- yl-amino)hexyl]-3-(1H-Indol-3-yl)propionamide (6T6BA) (IC50=11.0 nmol·L-1, electrophorus).
GUO Yong-Biao
,
LIU Hai-Bo
,
XU Ming
. Synthesis and Bioactivity of Novel Tacrine-Indole Hybrids by Microwave-Assisted Huisgen [3+2] Cycloaddition Reaction[J]. Chinese Journal of Organic Chemistry, 2012
, 32(02)
: 413
-419
.
DOI: 10.6023/cjoc1105143
[1] Hu, M.; Li, J.; Yao, A. Q. Org. Lett. 2008, 10(24), 5529.
[2] Ankati, H.; Biehl, E. Tetrahedron Lett. 2009, 50, 4677.
[3] Zhang, F.; Moses, J. E. Org. Lett. 2009, 11(7), 1587.
[4] Lewis, W. G.; Green, L. G.; Grynszpan, F.; Radi?, Z.; Carlier P. R.; Taylor, P.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41(6), 1053.
[5] Manetsch, R.; Krasinski, A.; Radic, Z.; Raushel, J.; Taylor, P.; Sharpless, K. B.; Kolb, H. C. J. Am. Chem. Soc. 2004, 126, 12809.
[6] Krasinski, A.; Radic, Z.; Manetsch, R.; Raushel, J.; Taylor, P.; Sharpless, K. B.; Kolb, H. C. J. Am. Chem. Soc. 2005, 127, 6686.
[7] Bourne, Y.; Kolb, H. C.; Radi?, Z.; Sharpless, K. B.; Taylor, P.; Marchot, P. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 1449.
[8] Muñoz-Ruiz, P.; Rubio, L.; García-Palomero, E.; Dorronsoro, I.; Monte-Millán, M.; Valenzuela, R.; Usán, P.; Austria, C.; Bartolini, M.; Andrisano, V.; Bidon-Chanal, A.; Orozco, M.; Luque, F. J.; Medina, M.; Martínez, A. J. Med. Chem. 2005, 48, 7223.
[9] Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004.
[10] Lourëat, F.; Bougrin, K.; Loupy, A.; Ochoa de Retana, A. M.; Pagalday, J.; Palacios, F. Heterocycles 1998, 48, 161.
[11] Katritzky, A. R.; Singh, S. K. J. Org. Chem. 2002, 67, 9077
[12] Balducci, E.; Bellucci, L.; Petricci, E.; Taddei, M.; Tafi, A. J. Org. Chem. 2009, 74, 1314.
[13] Guo, Y.-B.; Liu, H.-B.; Xu, M. Chin. J. Synth. Chem. 2011, 19(3), 299 (in Chinese). (郭永彪, 刘海波, 许明, 合成化学, 2011, 19(3), 299.)
[14] Ellman, G. L.; Courtney, K. D.; Andres, V. J.; Featherstone, R. M. Biochem. Pharm. 1961, 7, 88.