

Chinese Journal of Organic Chemistry >
2012 >Issue 03: 560 - 566
Synthesis and Biological Activities of Natural Flavonoid Diosmetin and Its Derivatives
Received date: 2011-09-08
Revised date: 2011-10-14
Online published: 2012-03-24
Supported by
Project supported by the Science and Technology Planning Project of Hunan Province (No. 2011FJ3214).
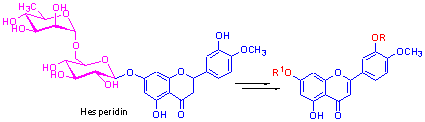
Key words: flavonoid; hesperidin; diosmetin; synthesis; biological activity
Cai Shuanglian , Wu Zheng , Wu Jing , Wang Qiuan , Shan Yang . Synthesis and Biological Activities of Natural Flavonoid Diosmetin and Its Derivatives[J]. Chinese Journal of Organic Chemistry, 2012 , (03) : 560 -566 . DOI: 10.6023/cjoc1109081





/
| 〈 |
|
〉 |