

Chinese Journal of Organic Chemistry >
Progress in Petasis Reaction
Received date: 2012-02-09
Revised date: 2012-04-19
Online published: 2012-05-21
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 20802088, 91017006, 90917017).
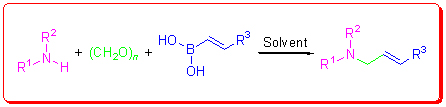
Petasis reaction is a powerful method for the synthesis of α-amino acids, β-amino alcohols and their derivatives. Chiral Petasis reaction has been applied in synthesis of nature products and drugs. This review describes the mechanism, reaction components and reaction conditions of Petasis reaction, and the applications of Petasis reaction are also discussed in the paper.
Yu Tao , Li Hui , Wu Xinyan , Yang Jun . Progress in Petasis Reaction[J]. Chinese Journal of Organic Chemistry, 2012 , 32(10) : 1836 -1845 . DOI: 10.6023/cjoc1202092
[1] Zhu, J.; Bienaymé, H. Multicomponent Reactions, Wiley-VCH, Weineim, Germany, 2005, pp. 206~223.
[2] Portlock, D. E.; Ostaszewski, R.; Naskar, D.; West, L. Tetrahedron Lett. 2003, 44, 603.
[3] Kalinski, C.; Lemoine, H.; Schmidt, J.; Burdack, C. Synthesis 2008, 4007.
[4] Jiang, B.; Xu, M. Angew. Chem., Int. Ed. 2004, 43, 2543.
[5] McReynolds, M. D.; Hanson, P. R. Chemtracts 2001, 14, 796.
[6] Petasis, N. A.; Akritopoulou, I. Tetrahedron Lett. 1993, 34, 583.
[7] Hall, D. Boronic Acid: Preparation and Applications in Organic Synthesis and Medicine, Wiley-VCH, Weinheim, Germany, 2005, pp. 279~304.
[8] Candeias, N. R.; Montalbano, F.; Cal, P. M. S. D.; Gois, P. M. P. Chem. Rev. 2010, 110, 6169.
[9] Hansen, T. K.; Schlienger, N.; Bryce, M. R. Tetrahedron Lett. 1999, 40, 3651.
[10] Jiang, B.; Xu, M. Org. Lett. 2002, 4, 4077.
[11] Neogi, S.; Roy, A.; Naskar, D. J. Comb. Chem. 2010, 12, 75.
[12] Au, C. W. G.; Nash, R. J.; Pyne, S. G. Chem. Commun. 2010, 46, 713.
[13] Schlienger, N.; Bryce, M. R.; Hansen, T. K. Tetrahedron 2000, 56, 10023.
[14] Tao, J.; Li, S. Chin. J. Chem. 2010, 28, 41.
[15] Kaiser, P. F.; Churches, Q. I.; Hutton, C. A. Aust. J. Chem. 2007, 60, 799.
[16] Petasis, N. A. Aust. J. Chem. 2007, 60, 795.
[17] Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457.
[18] Petasis, N. A.; Zavialov, I. A. J. Am. Chem. Soc. 1997, 119, 445.
[19] Charville, H.; Jackson, D.; Hodges, G.; Whiting, A. Chem. Commun. 2010, 46, 1813.
[20] Al-Zoubi, R. M.; Marion, O.; Hall, D. G. Angew. Chem., Int. Ed. 2008, 47, 2876.
[21] Ishihara, K.; Ohara, S.; Yamamoto, H. J. Org. Chem. 1996, 61, 4196.
[22] Petasis, N. A.; Patel, Z. D. Tetrahedron Lett. 2000, 41, 9607.
[23] Machan, T.; Davis, A. S.; Liawruangrath, B.; Pyne, S. G. Tetrahedron 2008, 64, 2725.
[24] Petasis, N. A.; Goodman, A.; Zavialov, I. A. Tetrahedron 1997, 53, 16463.
[25] Berrée , F.; Debache, A.; Marsac, Y.; Carboni, B. Tetrahedron Lett. 2001, 42, 3591.
[26] Jiang, B.; Yang, C. G.; Gu, X. H. Tetrahedron Lett. 2001, 42, 2545.
[27] Voisin, A. S.; Bouillon, A.; Lancelot, J.-C.; Lesnard, A.; Oulyadi, H.; Rault, S. Tetrahedron Lett. 2006, 47, 2165.
[28] Kolomeitsev, A. A.; Kadyrov, A. A.; Szczepkowska-Sztolcman, J.; Milewska, M.; Koroniak, H.; Bissky, G.; Barten, J. A.; Roeschenthaler, G.-V. Tetrahedron Lett. 2003, 44, 8273.
[29] Koolmeister, T.; Sodergren, M.; Scobie, M. Tetrahedron Lett. 2002, 43, 5965.
[30] Koolmeister, T.; Sodergren, M.; Scobie, M. Tetrahedron Lett. 2002, 43, 5969.
[31] Jourdan, H.; Gouhier, G.; Van Hijfte, L.; Angibaud, P.; Piettre, S. R. Tetrahedron Lett. 2005, 46, 8027.
[32] Stas, S.; Tehrani, K. A.; Laus, G. Tetrahedron 2008, 64, 3457.
[33] Morgan, I. R.; Yazici, A.; Pyne, S. G. Tetrahedron 2008, 64, 1409.
[34] Tremblay-Morin, J. P.; Raeppel, S.; Gaudette, F. Tetrahedron Lett. 2004, 45, 3471.
[35] Stas, S.; Tehrani, K. A. Tetrahedron 2007, 63, 8921.
[36] Konev, A. S.; Stas, S.; Novikov, M. S.; Khlebnikov, A. F.; Tehrani, K. A. Tetrahedron 2008, 64, 117.
[37] Tehrani, K. A.; Stas, S.; Lucas, B.; Kimpe, N. D. Tetrahedron 2009, 65, 1957.
[38] Petasis, N. A.; Zavialov, I. A. J. Am. Chem. Soc. 1998, 120, 11798.
[39] Naskar, D.; Roy, A.; Seibel, W. L.; Portlock, D. E. Tetrahedron Lett. 2003, 44, 5819.
[40] Hong, Z.; Liu, L.; Sugiyama, M.; Fu, Y.; Wong, C.-H. J. Am. Chem. Soc. 2009, 131, 8352.
[41] Chang, Y. M.; Lee, S. H.; Nam, M. H.; Cho, M. Y.; Park, Y. S.; Yoon, C. M. Tetrahedron Lett. 2005, 46, 3053.
[42] Portlock, D. E.; Naskar, D.; West, L.; Li, M. Tetrahedron Lett. 2002, 43, 6845.
[43] Naskar, D.; Roy, A.; Seibel, W. L.; Portlock, D. E. Tetrahedron Lett. 2003, 44, 8865.
[44] Naskar, D.; Neogi, S.; Roy, A.; Mandal, A. B. Tetrahedron Lett. 2008, 49, 6762.
[45] Naskar, D.; Roy, A.; Seibel, W. L. Tetrahedron Lett. 2003, 44, 8861.
[46] Wang, Q.; Finn, M. G. Org. Lett. 2000, 2, 4063.
[47] Berree, F.; Debache, A.; Marsac, Y.; Collet, B.; Girard-Le Bleiz, P.; Carboni, B. Tetrahedron 2006, 62, 4027.
[48] Southwood, T. J.; Curry, M. C.; Hutton, C. A. Tetrahedron 2006, 62, 236.
[49] Churches, Q. I.; Stewart, H. E.; Cohen, S. B.; Shroder, A.; Turner, P.; Hutton, C. A. Pure Appl. Chem. 2008, 80, 687.
[50] Shevchuk, M. V.; Sorochinsky, A. E.; Khilya, V. P.; Romanenko, V. D.; Kukhar, V. P. Synlett 2010, 73.
[51] Golebiowski, A.; Klopfenstein, S. R.; Chen, J. J.; Shao, X. Tetrahedron Lett. 2000, 41, 4841.
[52] Font, D.; Heras, M.; Villalgordo, J. M. Tetrahedron 2008, 64, 5226.
[53] Prakash, G. K. S.; Mandal, M.; Schweizer, S.; Petasis, N. A.; Olah, G. A. Org. Lett. 2000, 2, 3173.
[54] Prakash, G. K. S.; Mandal, M.; Schweizer, S.; Petasis, N. A.; Olah, G. A. J. Org. Chem. 2002, 67, 3718.
[55] Petasis, N. A.; Boral, S. Tetrahedron Lett. 2001, 42, 539.
[56] Petasis, N. A.; Butkevich, A. N. J. Organomet. Chem. 2009, 694, 1747.
[57] Grigg, R.; Sridharan, V.; Thayaparan, A. Tetrahedron Lett. 2003, 44, 9017.
[58] Kabalka, G. W.; Venkataiah, B.; Dong, G. Tetrahedron Lett. 2004, 45, 729.
[59] Kumagai, N.; Muncipinto, G.; Schreiber, S. L. Angew. Chem., Int. Ed. 2006, 45, 3635.
[60] Lou, S.; Schaus, S. E. J. Am. Chem. Soc. 2008, 130, 6922.
[61] Nanda, K. K.; Wesley Trotter, B. Tetrahedron Lett. 2005, 46, 2025.
[62] Yadav, J. S.; Reddy, B. V. S.; Lakshmi, P. N. J. Mol. Catal. A: Chem. 2007, 274, 101.
[63] Candeias, N. R.; Veiros, L. F.; Afonso, C. A. M.; Gois, P. M. P. Eur. J. Org. Chem. 2009, 1859.
[64] Candeias, N. R.; Cal, P. M. S. D.; Andre, V.; Duarte, M. T.; Gois, P. M. P. Tetrahedron 2010, 66, 2736.
[65] Klopfenstein, S. R.; Chen, J. J.; Golebiowski, A.; Li, M.; Peng, S. X.; Shao, X. Tetrahedron Lett. 2000, 41, 4835.
[66] Danieli, E.; Trabocchi, A.; Menchi, G.; Guarna, A. Eur. J. Org. Chem. 2007, 1659.
[67] Mieczkowski, A.; Kozmiński, W.; Jurczak, J. Synthesis 2010, 221.
[68] Thompson, K. A.; Hall, D. G. Chem. Commun. 2000, 2379.
[69] Regnier, T.; Berree, F.; Lavastre, O.; Carboni, B. Green Chem. 2007, 125.
[70] McLean, N. J.; Tye, H.; Whittaker, M. Tetrahedron Lett. 2004, 45, 993.
[71] Follmann, M.; Graul, F.; Schaefer, T.; Kopec, S.; Hamley, P. Synlett 2005, 1009.
[72] Sugiyama, S.; Arai, S.; Kiriyama, M.; Ishii, K. Chem. Pharm. Bull. 2005, 53, 100.
[73] Sugiyama, S.; Arai, S.; Ishii, K. Tetrahedron: Asymmetry 2004, 15, 3149.
[74] Neogi, S.; Roy, A.; Naskar, D. J. Comb. Chem. 2010, 12, 617.
[75] Davis, A. S.; Pyne, S. G.; Skelton, B. W.; White, A. H. J. Org. Chem. 2004, 69, 3139.
[76] Ritthiwigrom, T.; Pyne, S. G. Org. Lett. 2008, 10, 2769.
[77] Ritthiwigrom, T.; Willis, A. C.; Pyne, S. G. J. Org. Chem. 2010, 75, 815.
[78] Hong, Z.; Liu, L.; Hsu, C. C.; Wong, C. H. Angew. Chem., Int. Ed. 2006, 45, 7417.
[79] Au, C. W. G.; Pyne, S. G. J. Org. Chem. 2006, 71, 7097.
[80] Naskar, D.; Roy, A.; Seibel, W. L.; West, L.; Portlock, D. E. Tetrahedron Lett. 2003, 44, 6297.
[81] Portlock, D. E.; Naskar, D.; West, L.; Ostaszewski, R.; Chen, J. J. Tetrahedron Lett. 2003, 44, 5121.
/
| 〈 |
|
〉 |