

Chinese Journal of Organic Chemistry >
A Novel Strategy for the Synthesis of Benzofuran Derivatives
Received date: 2012-05-22
Revised date: 2012-06-03
Online published: 2012-06-06
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21072031, 20802009), the Ph.D. Programs Foundation of Chinese Ministry of Education (No. 200802461011) and the Shanghai Natural Science Foundation (No. 10ZR1404100).
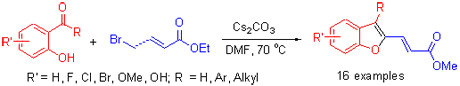
A novel method for the synthesis of benzofuran analogues via condensation of 2-hydroxybenzaldehyde or 2-hydroxyphenyl ketone with methyl 4-bromocrotonate was reported. Moderate to good yield was obtained with cesium carbonate as base in DMF at 70 ℃. The new-generated double bond was (E)-configuration determined by X-ray diffraction. All benzofuran derivatives were determined by 1H NMR, 13C NMR and HRMS techniques.
Zhao Yunhui , Liu Wenjie , Sun Xingwen , Lin Guoqiang . A Novel Strategy for the Synthesis of Benzofuran Derivatives[J]. Chinese Journal of Organic Chemistry, 2012 , 32(10) : 1919 -1924 . DOI: 10.6023/cjoc201205028
[1] (a) Horton, D. A.; Bourne, G. T.; Smythe, M. L. Chem. Rev. 2003, 103, 893.
(b) Donnelly, D. M. X.; Meegnan, M. In Comprehensive Heterocyclic Chemistry, Vol. 4, Ed.: Katrizky, A. R., Pergamon Press, New York, 1984, p. 657.
(c) Cagninant, P.; Cagninant, D. Adv. Heterocycl. Chem. 1975, 18, 337.
[2] (a) Erber, S.; Ringshandl, R.; von Angerer, E. Anti-Cancer Drug Des. 1991, 6, 417.
(b) Yoo, S.; Kim, S.-K.; Lee, S.-H.; Kim, N.-J.; Lee, D.-W. Bioorg. Med. Chem. 2000, 8, 2311.
(c) Gangjee, A.; Devraj, R.; Mcguire, J. J.; Kisliuk, R. L. J. Med. Chem. 1995, 38, 3798.
(d) Malamas, M. S.; Sredy, J.; Moxham, C.; Katz, A.; Xu, W.; McDevitt, R.; Adebayo, F. O.; Sawick, D. R.; Seestauer, L.; Sullivan, D.; Taylor, J. R. J. Med. Chem. 2000, 43,1293.
(e) Ebiike, H.; Masubuchi, M.; Liu, P.; Kawasaki, K.; Morikami, K.; Sogabe, S.; Hayase, M.; Fujii, T.; Sakata, K.; Shindoh, H.; Shiratori, Y.; Aoki, Y.; Ohtsuka, T.; Shimma, N. Bioorg. Med. Chem. Lett. 2002, 12, 607.
(f) Kawasaki, K.; Masabuchi, M.; Morikami, K.; Sogabe, S.; Aoyama, T.; Ebiike, H.; Niizuma, S.; Hayase, M.; Fujii, T.; Sakata, K.; Shindoh, H.; Shiratori, Y.; Aoki, Y.; Ohtsuka, T.; Shimma, N. Bioorg. Med. Chem. Lett. 2003, 13, 87.
(g) Masabuchi, M.; Kawasaki, K.; Ebiike, H.; Ikeda, Y.; Tsujii, S.; Sogabe, S.; Fujii, T.; Sakata, K.; Shiratori, Y.; Aoki, Y.; Ohtsuka, T.; Shimma, N. Bioorg. Med. Chem. Lett. 2001, 11, 1833.
(h) Masubuchi, M.; Ebiike, H.; Kawasaki, K.; Sogabe, S.; Morikami, K.; Shiratori, Y.; Tsujii, S.; Fujii, T.; Sakata, K.; Hayase, M.; Shindoh, H.; Aoki, Y.; Ohtsuka, T.; Shimma, N. Bioorg. Med. Chem. 2003, 11, 4463.
[3] (a) Cacchi, S.; Fabrizi, G.; Goggiomani, A. Heterocycles 2002, 56, 613.
(b) Cacchi, S.; Fabrizi, G.; Goggiamani, A. Curr. Org. Chem. 2006, 10, 1423.
(c) Hou, X.-L.; Yang, Z.; Yeung, K.-S.; Wong, H. N. C. Prog. Heterocycl. Chem. 2007, 18, 187.
(d) Byun, J. H.; Kim, H. Y.; Kim, Y. S.; Mook-Jung, I.; Kim, D. J.; Lee, W. K.; Yoo, K. H. Bioorg. Med. Chem. Lett. 2008, 18, 5591.
(e) Patil, S. A.; Patil, R.; Miller, D. D. Curr. Med. Chem. 2009, 16, 2531.
(f) De Luca, L.; Nieddu, G.; Porcheddu, A.; Giacomelli, G. Curr. Med. Chem. 2009, 16, 1.
(g) Suh, J.; Yi, K. Y.; Lee, Y.-S.; Kim, E.; Yum, E. K.; Yoo, S.-E. Bioorg. Med. Chem. Lett. 2010, 20, 6362.
[4] Chen, Y.; Chen, S.; Lu, X.; Cheng, H.; Ou, Y.; Cheng, H.; Zhou, G.-C. Bioorg. Med. Chem. Lett. 2009, 19, 1851.
[5] Geary, L. M.; Hultin, P. G. Eur. J. Org. Chem. 2010, 5563.
[6] (a) Tsai, T.-W.; Wang, E.-C.; Huang, K.-S.; Li, S.-R.; Wang, Y.-F.; Lin, Y.-L.; Chen, Y.-H. Heterocycles 2004, 63, 1771.
(b) van Otterlo, W. A. L.; Morgans, G. L.; Madeley, L. G.; Kuzvidza, S.; Moleele, S. S.; Thornton, N.; de Koning, C. B. Tetrahedron 2005, 61, 7746.
[7] (a) De Luca, L.; Giacomelli, G.; Nieddu, G. J. Org. Chem. 2007, 72, 3955.
(b) De Luca, L. Giacomelli, G. Nieddu, G. J. Comb. Chem. 2008, 10, 517.
[8] (a) Willis, M. C.; Taylor, D.; Gillmore, A. T. Org. Lett. 2004, 6, 4755.
(b) Willis, M. C.; Taylor, D.; Gillmore, A. T. Tetrahedron 2006, 62, 11513.
(c) Anderson, K. W.; Ikawa, T.; Tundel, R. E.; Buchwald, S. L. J. Am. Chem. Soc. 2006, 128, 10694.
(d) Ackermann, L.; Kaspar, L. T. J. Org. Chem. 2007, 72, 6149.
(e) Carril, M.; SanMartin, R.; Tellitu, I.; Dominguez, E. Org. Lett. 2006, 8, 1467.
(f) Lu, B.; Wang, B.; Zhang, Y.; Ma, D. J. Org. Chem. 2007, 72, 5337.
[9] Farago, J.; Kotschy, A. Synthesis 2009, 85.
[10] (a) Guthrie, E. J.; Macritchie, J.; Hartley, R. C. Tetrahedron Lett. 2000, 41, 4987.
(b) Nicolaou, K. C.; Snyder, S. A.; Bigot, A.; Pfefferkorn, J. A. Angew. Chem., Int. Ed. 2000, 39, 1093.
(c) Chittimalla, S. K.; Chang, T.-C.; Liu, T.-C.; Hsieh, H.-P.; Liao, C.-C. Tetrahedron 2008, 64, 2586.
(d) Sales, Z. S.; Mani, N. S. J. Org. Chem. 2009, 74, 891.
[11] (a) Zeni, G.; Larock, R. C. Chem. Rev. 2004, 104, 2285.
(b) Fuerstner, A.; Davies, P. W. J. Am. Chem. Soc. 2005, 127, 15024.
(c) Yue, D.; Yao, T.; Larock, R. C. J. Org. Chem. 2005, 70, 10292.
(d) Nakamura, I.; Mizushima, Y.; Yamamoto, Y. J. Am. Chem. Soc. 2005, 127, 15022.
(e) Grigg, R.; Sridharan, V.; Sykes, D. A. Tetrahedron 2008, 64, 8952.
(f) Isono, N.; Lautens, M. Org. Lett. 2009, 11, 1329.
[12] Guo, H.-F.; Shao, H.-Y.; Yang, Z.-Y.; Xue, S.-T.; Li, X.; Liu, Z.-Y.; He, X.-B.; Jiang, J.-D.; Zhang, Y.-Q.; Si, S.-Y.; Li, Z.-R. J. Med. Chem. 2010, 53, 1819.
[13] Matsunaga, N.; Kaku, T.; Itoh, F.; Tanaka, T.; Hara, T.; Miki, H.; Iwasaki, M.; Aono, T.; Yamaoka, M.; Kusaka, M.; Tasaka, A. Bioorg. Med. Chem. 2004, 12, 2251.
[14] (a) Han, X.; Ye, L.-W.; Sun, X.-L.; Tang, Y. J. Org. Chem. 2009, 74, 3394.
(b) Li, N.; Song, J.; Tu, X.-F.; Liu, B.; Chen, X.-H.; Gong, L.-Z. Org. Biomol. Chem. 2010, 8, 2016.
/
| 〈 |
|
〉 |