

Chinese Journal of Organic Chemistry >
An Investigation on the Transition Metal Catalyzed Selective Additions of Styrenes with Diacetoxyiodobenzene (DIB)- Tetrabutylamidebromide (TBAB) System
Received date: 2012-05-17
Revised date: 2012-06-05
Online published: 2012-06-11
Supported by
Project supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Opening Foundation of Zhejiang Provincial Top Key Discipline (No. 100061200138), the Natural Scientific Foundation of Jiangsu Province (No. BK2010321), the University Natural Scientific Foundation of Jiangsu Province (No. 09KJB150014) and the Opening Labortary Fundation of Jiangsu Province (No. K100027).
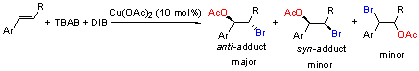
Key words: catalysis; transition metal; regioselecticvity; stereoselecticvity; addition
Fan Lei , Ren Lingfeng , Chen Tian , Yi Rong , Yu Lei . An Investigation on the Transition Metal Catalyzed Selective Additions of Styrenes with Diacetoxyiodobenzene (DIB)- Tetrabutylamidebromide (TBAB) System[J]. Chinese Journal of Organic Chemistry, 2012 , 32(08) : 1439 -1444 . DOI: 10.6023/cjoc201205023
/
| 〈 |
|
〉 |