

Chinese Journal of Organic Chemistry >
Synthesis, Crystal Structure, Antioxidant Activity of Chalcones and Its Spiro-heterocyclic Analogues
Received date: 2012-04-01
Revised date: 2012-06-18
Online published: 2012-07-02
Supported by
Project supported by the Technology Foundation for Medical Science of Zhejiang Province (No. 2012KYA129), the Project of Wenzhou Sci & Tech Bureau (No. 20090101), and the Technology Foundation for Chinese Medicine of Zhejiang Province (No. 2012ZB102).
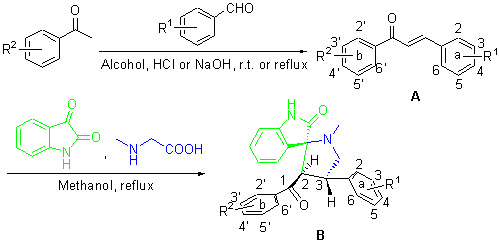
To synthesize new structure type chalcone analogues, and obtain chalcone analogues with good antioxidant activity. Two types of chalcone analogs, chalcones A and spiro-heterocyclic B were designed and synthesized, and the structures of 21 compounds were characterized by 1H NMR, ESI-MS and ESI-HRMS. Single-crystal of spiro-heterocyclic B was cultured, and its single-crystal structure was determined by X-ray diffraction study. The crystal structure of B1 was monoclinic system, space group C2/c, with cell dimensions of a=21.350(3) Å, b=8.6256(10) Å, c=26.161(3) Å. Spiro-heterocyclic B is the new structure type, and obtained by one-pot synthesis which is 1,3-dipolar cycloaddition reaction and no catalyst. The synthesis of spiro-heterocyclic B is not only high regioselectivity and stereoselectivity, but also environmentally friendly. The antioxidant activities in vitro were evaluated by 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay. Many compounds, which can have strong activity of scavenging DPPH free radicals, were screened. In the 2 types of chalcones analogs, the compounds with 3,4-(OH)2 in “a” ring have excellent antioxidant activities. The chalcone analogs with o-dihydroxy in benzene ring maybe have good antioxidant activities.
Key words: chalcones; spiro-heterocyclic; synthesis; DPPH; antioxidant activities; crystal structure
Wu Jianzhang , Li Wulan , Chen Lingzi , Chu Shenghui , Zhao Chenguang , Wei Tao , Yang Shulin , Li Xiaokun . Synthesis, Crystal Structure, Antioxidant Activity of Chalcones and Its Spiro-heterocyclic Analogues[J]. Chinese Journal of Organic Chemistry, 2012 , 32(11) : 2141 -2147 . DOI: 10.6023/cjoc201204001
/
| 〈 |
|
〉 |