

Chinese Journal of Organic Chemistry >
Synthesis of 4-Hydroxy-6-methyl-[(1-phenylimino)ethyl]-2H-pyran-2- one Derivatives Catalyzed by Ionic Liquid [BMIM-HSO3]HSO4 under Ultrasound
Received date: 2012-04-18
Revised date: 2012-06-19
Online published: 2012-07-02
Supported by
Project supported by the Natural Science Foundation of Tianjin City (No. 11JCZDJC21300).
4-Hydroxy-6-methyl-[(1-phenylimino)ethyl)]-2H-pyran-2-one derivatives have been synthesized via the reaction of dehydroacetic acid and amine in the presence of the ionic liquid [BMIM-HSO3]HSO4 under ultrasonic irradiation. The effect of catalyst, catalyst amount, reaction temperature and solvent on the yield was investigated. The optimum reaction condition was determined and the possible mechanism was proposed. This method has some distinct advantages such as short reaction time, high yields, environmental friendliness and mild reaction conditions. Moreover, the ionic liquid could be recovered conveniently and reused.
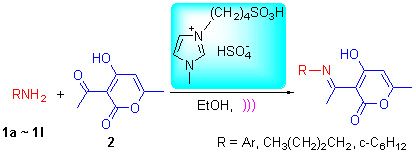
Key words: ionic liquid; ultrasound; dehydroacetic acid; amine
Li Daqing , Zang Hongjun , Wu Changchun , Lu Bo , Cheng Bowen . Synthesis of 4-Hydroxy-6-methyl-[(1-phenylimino)ethyl]-2H-pyran-2- one Derivatives Catalyzed by Ionic Liquid [BMIM-HSO3]HSO4 under Ultrasound[J]. Chinese Journal of Organic Chemistry, 2012 , 32(11) : 2193 -2197 . DOI: 10.6023/cjoc201204020
/
| 〈 |
|
〉 |