

Chinese Journal of Organic Chemistry >
Synthesis of 2-Ester-substituted-1,5-benzoxazepines and Studies on the By-product in the Reaction
Received date: 2012-05-29
Revised date: 2012-07-11
Online published: 2012-07-17
Supported by
Project supported by the National Natural Science Foundation of China (No. 20972040).
A series of new 1,5-benzoxazepines have been synthesized from substituted benzene, maleic anhydride and 2-aminophenol (4-chloro-2-aminophenol) and identified by IR, 1H NMR, MS (HRMS) techniques and elemental analysis. Meanwhile, the structure of one main by-product 2-benzoylmethyl-2H-1,4-benzoxazin-3(4H)-one (6h') was identified, and the possible mechanism of producing the by-product was proposed. The optimization of reaction conditions showed that the reactions were performed in DMF solvent, at refluxed temperature and catalyzed by p-toluenesulfonic acid, and 2-ester-sub-stituted-1,5-benzoxazepines 6 were the main products. While, the reactions were performed in methyl alcohol, at refluxed temperature and catalyzed by acetic acid, and the by-product 6h' was formed in good yield.
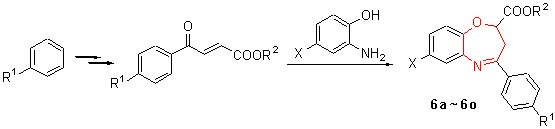
Liu Qian , Zhao Lifei , Li Wenhong , Li Yuan . Synthesis of 2-Ester-substituted-1,5-benzoxazepines and Studies on the By-product in the Reaction[J]. Chinese Journal of Organic Chemistry, 2012 , 32(11) : 2122 -2128 . DOI: 10.6023/cjoc201205034
/
| 〈 |
|
〉 |