

Chinese Journal of Organic Chemistry >
Synthesis and Protein Tyrosine Phosphatase 1B Inhibitory Activity of Novel Chalcone Derivatives Containing 3,4-Dihydroquinolin-2(1H)-one Moiety
Received date: 2012-06-18
Revised date: 2012-07-16
Online published: 2012-07-19
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 20962021, 81125023).
Protein tyrosine phosphatase 1B (PTP1B) has recently been identified as new drug target for type II diabetes and obesity due to it is a negative regulator of the insulin and leptin-signaling pathway. In order to find new nonphosphonate-based pTyr mimetics, a series of novel chalcone derivatives bearing the 3,4-dihydroquinolin-2(1H)-one moieties (4a~4n) were designed, synthesized, and evaluated for their PTP1B inhibitory activity. The results demonstrated that all compounds presented potent inhibitory activities against PTP1B, among which compounds 4e and 4i exhibited most potent with IC50 value of (4.64±0.38) and (4.36±0.41) μmol/L, respectively.
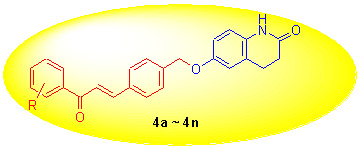
Key words: chalcone; 3,4-dihydroquinolin-2(1H)-one; PTP1B inhibitor
Sun Liangpeng , Jiang Zhe , Gao Lixin , Li Yingzhe , Sun Liyun , Li Jia , Piao Huri . Synthesis and Protein Tyrosine Phosphatase 1B Inhibitory Activity of Novel Chalcone Derivatives Containing 3,4-Dihydroquinolin-2(1H)-one Moiety[J]. Chinese Journal of Organic Chemistry, 2012 , 32(11) : 2108 -2114 . DOI: 10.6023/cjoc201206018
[1] (a) Neel, B. G.; Tonks, N. K. Curr. Opin. Cell Biol. 1997, 9, 193.
(b) Hunter, T. Cell 2000, 100, 113.
(c) Tonks, N. K.; Neel, B.G. Curr. Opin. Cell Biol. 2001, 13, 182.
[2] (a) Elchebly, M.; Payette, P.; Michaliszyn, E.; Cromlish, W.; Collins, S.; Loy, A. L.; Normandin, D.; Cheng, A.; Himms-Hagen, J.; Chan C. C.; Ramachandran, C.; Gresser, M. J.; Tremblay, M. L.; Kennedy, B. P. Science 1999, 283, 1544.
(b) Klaman, L. D.; Boss, O.; Peroni, O. D.; Kim, J. K.; Martino, J. L.; Zabolotny, J. M.; Moghal, N.; Lubkin, M.; Kim, Y. B.; Sharpe, A. H.; Stricker-Krongrad, A.; Shulman, G. I.; Neel, B. G.; Kahn, B. B. Mol. Cell Biol. 2000, 20, 5479.
[3] Zinker, B. A.; Rondinone, C. M.; Trevillyan, J. M.; Gum, R. J.; Clampit, J. E.; Waring, J. F.; Xie, N.; Wilcox, D.; Jacobson, P.; Frost, L.; Kroeger, P. E.; Reilly, R. M.; Koterski, S.; Opgenorth, T. J.; Ulrich, R. G.; Crosby, S.; Butler, M.; Murray, S. F.; McKay, R. A.; Bhanot, S.; Monica, B. P.; Jirousek, M. R. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 11357.
[4] Rondinone, C. M.; Trevillyan, J. M.; Clampit, J. E.; Gum, R. J.; Berg, C.; Kroeger, P.; Frost, L.; Zinker, B. A.; Reilly, R.; Ulrich, R.; Butler, M.; Monia, B. P.; Jirousek, M. R.; Waring, J. F. Diabetes 2002, 51, 2405.
[5] Ramachandran, C.; Kennedy, B. P. Curr. Top Med. Chem. 2003, 3, 749.
[6] Combs, A. P. J. Med. Chem. 2010, 53, 2333.
[7] Vogel, S.; Ohmayer, S.; Brunner, G.; Heilmann, J. Bioorg. Med. Chem. 2008, 16, 4286.
[8] (a) Chen, Z. H.; Zheng, C. J.; Sun, L. P.; Piao, H. R. Eur. J. Med. Chem. 2010, 45, 5739.
(b) Liu, X. F.; Zheng, C. J.; Sun, L. P.; Liu, X. K.; Piao, H. R. Eur. J. Med. Chem. 2011, 46, 3469.
[9] Chiaradia, L. D.; dos Santos, R.; Vitor, C. E.; Vieira, A. A.; Leal, P. C.; Nunes, R. J.; Calixto, J. B.; Yunes, R. A. Bioorg. Med. Chem. 2008, 16, 658.
[10] Vogel, S.; Heilmann, J. J. Nat. Prod. 2008, 71, 1237.
[11] Domínguez, J. N.; León, C.; Rodrigues, J.; Gamboa de Domínguez, N.; Gut, J.; Rosenthal, P. J. J. Med. Chem. 2005, 48,3654.
[12] Daskiewicz, J. B.; Depeint, F.; Viornery, L.; Bayet, C.; Comte-Sarrazin, G.; Comte, G.; Gee, J. M.; Johnson, I. T.; Ndjoko, K.; Hostettmann, K.; Barron, D. J. Med. Chem. 2005, 48, 2790.
[13] Sui, X.; Quan, Y. C.; Chang, Y.; Zhang, R. P.; Xu, Y. F.; Guan, L. P. Med. Chem. Res. 2011, 20, 1.
[14] Chen, R. M.; Hu, L. H.; An, T. Y.; Li, J.; Shen, Q. Bioorg. Med. Chem. Lett. 2002, 12, 3387.
[15] Yoon, G.; Lee, W.; Kim, S. N.; Cheon, S. H. Bioorg. Med. Chem. Lett. 2009, 19, 5155.
[16] Liu, Z.; Lee, W.; Kim, S. N.; Yoon, G.; Cheon, S. H. Bioorg. Med. Chem. Lett. 2011, 21, 3755.
[17] Ge, H. X.; Wang, L. C. Prog. Pharm. Sci. 2005, 29, 310 (in Chinese).
(葛海霞, 王礼琛, 药学学报, 2005, 29, 310.)
[18] Patil, V.; Tilekar, K.; Mehendale-Munj, S.; Mohan, R.; Ramaa, C. S. Eur. J. Med. Chem. 2010, 49, 4539.
[19] Meng, F. L.; Zheng, C. J.; Li, Y. J.; Sun, L. P.; Liu, X. K.; Zhang, T. Y.; Piao, H. R. Chin. J. Org. Chem. 2012, 32, 183 (in Chinese).
(孟凡领, 郑昌吉, 李因晶, 孙良鹏, 刘学坤, 张天一, 朴虎日, 有机化学, 2012, 32, 183.)
[20] (a) Shi, L.; Yu, H. P.; Zhou, Y. Y.; Du, J. Q.; Shen, Q.; Li, J. Y.; Li, J. Acta Pharmacol. Sin. 2008, 29, 278.
(b) Zhang, W.; Hong, D.; Zhou, Y. Y.; Zhang, Y. N.; Shen, Q.; Li, J. Y.; Hu, L. H.; Li, J. Biochim. Biophys. Acta 2006, 1760, 1505.
/
| 〈 |
|
〉 |