

Chinese Journal of Organic Chemistry >
Synthesis and Study of Properties for Asymmetric Triphenylamine-Zinc Phthalocyanine
Received date: 2012-06-19
Revised date: 2012-08-01
Online published: 2012-08-10
Supported by
Project supported by the National Natural Science Foundation of China (No. 20871108) and the Natural Science Foundation of Shanxi Province (No. 2011011022-4).
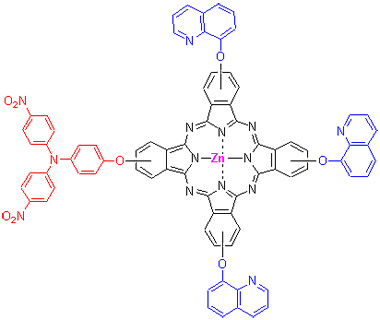
Triphenylamine-zinc phthalocyanine (TQPc) that contains a bulky triphenylamine group and three 8-oxy-quinoline groups has been found to exhibit preferable performance. The first example of TQPc has been synthesized. Its zinc complexes of the well-known electron transport material displays enhanced electron-accepting ability relative to free- metallophthalocyanines, and can also be used as chemical sensors, liquid crystals, photodynamic therapy, data storage and non-linear optics. The triphenylamine-zinc phthalocyanine is synthesized by statistical condensation. The main chemicals are aminophenol, p-chloronitrobenzene, 8-hydroxyquinoline, 4-nitrophthalonitrile and zinc acetate. The fragment 4,4'-dinitro-4"-hydroxy- triphenylamine is synthesized by the reacting from aminophenol with p-chloronitrobenzene at the catalysis of anhydrous potassium carbonate. The gross yield is 27.5%, and its purity is 96.6%. In comparison with other methods, there are some advantages in this method: the materials are cheap and easily available, and the product can be used as a substituent for phthalocyanine. All compounds are characterized by 1H NMR, IR spectra and elemental analysis. The electronic spectra of TQPc exhibits an intense π-π* transition of triphenylamine unit identity together with characteristic B bands of the phthalocyanine core. Energy transfer through oxy bridges has been confirmed by ultraviolet irradiation of triphenylamine. The aggregative behavior is studied in DMF and CH2Cl2. The results indicate that it is not aggregative in DMF, whereas forms dimer in CH2Cl2 at 0.223×10-5~2.587×10-5 mol/L. The equilibrium constant for the dimer is calculated at the same time, indicating that the less polar solvent is unfavorable for the presence of the monomer. The redox behavior is studied by cyclic voltammetry. Its level structure of energy band is calculated by cyclic voltammetry combined with differential voltammograms, this molecule has been found to have a low LUMO (-1.04 V vs SCE) and a deep HOMO (0.78 V vs SCE) energy level. The results indicate that it matches with the energy level of the nanocrystalline of TiO2, and is thus very promising as an electron transport material for dye-sensitized solar cells.
Key words: phthalocyanine; 8-hydroxyquinoline; triphenylamine; photosensitive dyes; dimer
Mao Lijun , Tan Qinglong , Xin Guanqiong , Han Mingliang , Zhang Xuejun . Synthesis and Study of Properties for Asymmetric Triphenylamine-Zinc Phthalocyanine[J]. Chinese Journal of Organic Chemistry, 2012 , 32(12) : 2315 -2321 . DOI: 10.6023/cjoc201206019
[1] O′regan, B.; Grätzel, M. Nature 1991, 353, 737.
[2] Hagfeldt, A.; Grätzel, M. Chem. Rev. 1995, 95, 49.
[3] Dai, S., Y.; Wang, K., J.; Weng, J.; Sui, Y., F.; Huang, Y.; Xiao, S., F. Solar Energy Mater. Solar Cells 2005, 85, 447.
[4] Horiuchi, T.; Miura, H.; Uchida, S. J. Photochem. Photobiol. A: Chem. 2004, 164, 29.
[5] Li, C., Y.; Yang, X., C.; Chen, R., K.; Pan, J., X; Tian, H., N.; Zhu, H., J.; Wang, X., N.; Hagfeldt, A.; Sun, L., C. Solar Energy Mater. Solar Cells 2007, 91, 1863.
[6] Qin, H.; Wenger, S.; Xu, M.; Gao, F.-F.; Jing, X.-Y.; Wang, P.; Zakeeruddin, S. M.; Grätzel, M. J. Am. Chem. Soc. 2008, 130, 9202.
[7] Dastoor, P. C.; McNeill, C. R.; Frohne, H.; Foster, C. J.; Dean, B.; Fell, C. J.; Belcher, W. J.; Campbell, W. M.; Officer, D. L.; Blake, L. M.; Thordarson, P.; Crossley, M. J.; Hush, N. S.; Reimers, J. R. J. Phys. Chem. C 2007, 111, 15415.
[8] Deng, H.-H.; Mao, H.-F.; Shen, Y.-C.; Lu, Z.-H.; Xu, H.-J. Acta Chim. Sinica 1999, 57, 1199 (in Chinese).
(邓慧华, 毛海舫, 沈耀春, 陆祖宏, 许慧君, 化学学报, 1999, 57, 1199.)
[9] Hou, Y. J.; Xie, P. H.; Zhang, B. W.; Cao, Y.; Xiao, X. R.; Wang, W. B. Inorg. Chem. 1999, 38, 6320.
[10] Clifford, J. N.; Palomares, E.; Nazeeruddin, M. D. K.; Thampi, R.; Grätzel, M.; Durrant, J. R. J. Am. Chem. Soc. 2004, 126, 5670.
[11] Kong, F.-T.; Dai, S.-Y.; Wang, K.-J. Chemistry 2005, (5), 338 (in Chinese).
(孔凡太, 戴松元, 王孔嘉, 化学通报, 2005, (5), 338.)
[12] Wu, D.; Shen, Z.; Xue, Z.-L.; You, X., Z. Chin. J. Inorg. Chem. 2007, 23, 1 (in Chinese).
(吴迪, 沈珍, 薛兆历, 游效曾, 无机化学学报, 2007, 23, 1.)
[13] Wang, Q.; Campbell, W. M.; Bonfantani, E. E.; Jolley, K. W.; Officer, D.; Walsh, P. J.; Gordon, K.; Baker, R. H.; Nazeeruddin, M. K.; Grätzel, M. J. Phys. Chem. B 2005, 109, 15397.
[14] Chen, W.; Duan, W.-B.; He, C.-Y.; Zuo, X.; Wu, Y.-Q. Chin. J. Inorg. Chem. 2005, 21, 1880 (in Chinese).
(陈伟, 段武彪, 贺春英, 左霞, 吴谊群, 无机化学学报, 2005, 21, 1880.)
[15] Hammer, R. P.; Owens, C. V.; Hwang, S. H.; Sayes, C. M.; Soper, S. A. Bioconjugate Chem. 2002, 13, 1244.
[16] Campbell, W. M.; Jolley, K. W.; Wagner, P.; Wagner, K.; Walsh, P. J.; Gordon, K. C.; Mende, L. S.; Nazeeruddin, M. K.; Wang, Q.; Grätzel, M.; Officer, D. L. J. Phys. Chem. C 2007, 111, 11760.
[17] Bai, Q.-L.; Zhang, C.-H.; Cheng, C.-H.; Li, W.-C.; Du, G.-T. Acta Chim. Sinica 2011, 69, 949 (in Chinese).
(白青龙, 张春花, 程传辉, 李万程, 杜国同, 化学学报, 2011, 69, 949.)
[18] Yu, X.-W.; Zhan, C.-L.; Huang, Y. Chin. J. Org. Chem. 2012, 32, 770 (in Chinese).
(俞孝伟, 詹传郎, 黄彦, 有机化学, 2012, 32, 770.)
[19] Zhang, D.; Zhang, X.-J.; Zhang, L.; Mao, L.-J. Bull. Korean Chem. Soc. 2012, 33, 1
[20] Pei, J. Ph.D. Dissertation, Nankai University, Tianjin, 2009 (in Chinese).
(裴娟, 博士论文, 南开大学, 天津, 2009.)
[21] Peng, Y.-R.; Huang, F.-H. Strait Pharm. J. 2003, 15, 53 (in Chinese).
(彭亦如, 黄风华, 海峡药学, 2003, 15, 53.)
[22] Liu, Q.-H.; Fu, Q.; Yang, J.; Ma, J.-C.; Li, W.-L.; Wang, X. J. Mol. Struct. 2010, 963, 41
[23] Yenilmez, H.-Y.; özcesmeci, ?.; Okur, A. ?.; Gül, A. Polyhedron 2004, 23, 787.
[24] Liu, L.-W. M.S. Thesis, Zhejiang University, Hangzhou, 2008 (in Chinese).
(刘立维, 硕士论文, 浙江大学, 杭州, 2008.)
[25] Li, W.-L. M.S. Thesis, Northeast Normal University, Changchun, 2010 (in Chinese).
(李伟利, 硕士论文, 东北师范大学, 长春, 2010.)
[26] Sastre, A.; Torres, T.; Hanack, M. Tetrahedron Lett. 1995, 36, 8501.
[27] Zhang, X.-J.; Mao, L.-J.; Zhang, D.; Zhang, L. J. Mol. Struct. 2012, 1022, 153.
[28] Giribabu, L.; Vijay, K. C.; Gopal, R. V.; Yella, R. P. Solar Energ. Mater. Solar Cells 2007, 91, 1611.
[29] Hagfeldt, A.; Grätzel, M. Chem. Rev. 1995, 95, 49.
[30] Liu, L.-W.; Shi, M.-M.; Deng, D.; Wang, M.; Chen, H.-Z. Acta Chim. Sinica 2008, 66, 2163 (in Chinese).
(刘立维, 施敏敏, 邓丹, 汪茫, 陈红征, 化学学报, 2008, 66, 2163.)
[31] Zhang, T.-L.; Yan, J.-M. Acta Chim. Sinica 2000, 58, 981 (in Chinese).
(张天莉, 严继民, 化学学报, 2000, 58, 981.)
/
| 〈 |
|
〉 |