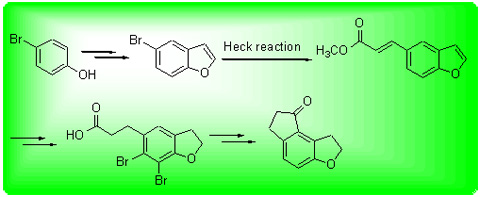
A novel process for synthesis of 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one, a key intermediate for preparation of ramelteon, a MT1 and MT2 melatonin receptor selective agonist, was developed. The key intermediate 3-(2,3-dihydrobenzofuran-5-yl)propanoic acid was synthesized by condensation of p-bromophenol with bromoacetaldehyde diethyl acetal in the presence of K2CO3 and Friedel-Crafts reaction to give 5-bromobenzofuran, which was subsequently subjected to Heck coupling reaction with methyl acrylate in the presence of palladium acetate, then catalytic hydrogenation and hydrolysis in one pot reaction. Subsequently, 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one was synthesized from 3-(2,3-dihydrobenzo- furan-5-yl)propanoic acid through bromination, Friedel-Crafts acylation and catalytic hydrogenolysis debromination. The overall yield was about 49.9%. The structures of intermediates and final product were determined by 1H NMR, 13C NMR and HRMS techniques. This method has the advantages of easily available starting materials, simply conducted procedures, relatively high yield and easy purification, and is more suitable for scale-up production.
Huang Zhixiong
,
Wu Chenglong
,
Shang Zhipei
,
Deng Yong
. An Improved Synthesis of 1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one[J]. Chinese Journal of Organic Chemistry, 2012
, 32(12)
: 2368
-2372
.
DOI: 10.6023/cjoc201207023
[1] Uchikawa, O.; Fukatsu, K.; Tokunoh, R.; Kawada, M.; Matsumoto, K.; Imai, Y.; Hinuma, S.; Kato, K.; Nishikawa, H.; Hirai, K.; Miyamoto, M.; Ohkawa, S. J. Med. Chem. 2002, 45, 4222.
[2] Simpson, D.; Curran, M. P. Drugs 2008, 68, 1901.
[3] Miyamoto, M. CNS Neurosci. Ther. 2009, 15, 32.
[4] (a) Kansal, V. K.; Mistry, D. N.; Vasoya, S. L. WO 2008151170, 2008 [Chem. Abstr. 2008, 150, 35215].
(b) Jiang, L.; Xia, Z. J.; Chen, Z. X.; Yao, C. Chin. J. Pharm. 2009, 40, 161 (in Chinese).
(蒋龙, 夏正君, 陈再新, 姚成, 中国医药工业杂志, 2009, 40, 161.)
(c) Urayama, S.; Mutou, E.; Inagaki, A.; Okada, T.; Sugisaki, S. WO 2006030739, 2006 [Chem. Abstr. 2006, 144, 331252].
[5] (a) Zhu, Y. W.; Wei, J. J.; Chen, L. WO 2011044990, 2011 [Chem. Abstr. 2011, 154, 486197].
(b) Wang, B.; Zhang, L. J.; Fu, K.Y. Org. Prep. Proced. Int. 2009, 41, 309.
(c) Cluzeau, J. WO 2010007022, 2010 [Chem. Abstr. 2010, 152, 191935].
(d) Cluzeau, J. WO 2010115897, 2010 [Chem. Abstr. 2010, 153, 505632].
[6] (a) Taveras, A. G.; Chao, J.; Biju, P. J.; Yu, Y.; Fine, J. S.; Hipkin, W.; Aki, C. J.; Merritt, J. R.; Li, G.; Baldwin, J. J.; Lai, G.; Wu, M.; Hecker, E. A WO 2004033440, 2004 [Chem. Abstr. 2004, 140, 357355].
(b) Dunn, R.; Xie, W.; Tehim, A. WO 2009023844, 2009 [Chem. Abstr. 2009, 150, 237586].
[7] Kangani, C. O.; Day, B. W. Org. Lett. 2008, 10, 2645.
[8] Huang, H. Y.; Ishikawa, T.; Peng, C. F.; Tsai, I. L.; Chen, I. S. J. Nat. Prod. 2008, 71, 1146.


