

Chinese Journal of Organic Chemistry >
Cesium Chloride Catalysized the Reaction of Sodium (Potassium) Carboxylates with Alkyl Bromides (Chlorides)
Received date: 2012-08-13
Revised date: 2012-09-08
Online published: 2012-09-21
Supported by
Project supported by the Natural Science Foundation of Hunan Province (No. 08JJ3018).
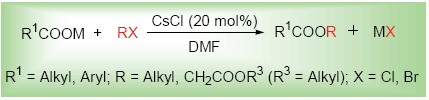
In the presence of catalytic amounts of cesium chloride, using DMF as solvent, sodium (potassium) carboxylates reacted with α-chloroacetates at room temperature or reacted with alkyl bromides (chlorides) at 60℃ to give the corresponding (α-alkoxyformacyl)methyl carboxylic esters and carboxylic esters in >90% yields. The catalytic mechanism was discussed.
Liu Wenqi , Qi Jianyong , Chen Jinyang , Li Ningbo , Qui Renhua , Xu Xinhua . Cesium Chloride Catalysized the Reaction of Sodium (Potassium) Carboxylates with Alkyl Bromides (Chlorides)[J]. Chinese Journal of Organic Chemistry, 2013 , 33(01) : 190 -194 . DOI: 10.6023/cjoc201208010
[1] Tzalis, D.; Knochel, P. Angew. Chem., Int. Ed. 1999, 38, 1463.
[2] Salvatore, R. N.; Nagle, A. S.; Jung, K. W. J. Org. Chem. 2002, 67, 674.
[3] Tzalis, D.; Koradin, C.; Knochel, P. Tetrahedron Lett. 1999, 40, 6193.
[4] Li, X. W.; Liu, W. Q.; Zou, K. B. Chin. J. Org. Chem. 2007, 27, 1176 (in Chinese). (黎小武, 刘文奇, 邹康兵, 许新华, 有机化学, 2007, 27, 1176.)
[5] Xia, X.; Zou, K. B.; Fang, D. W.; Xu, X. H. Chin. J. Org. Chem. 2008, 28, 1487 (in Chinese). (夏湘, 邹康兵, 方大为, 许新华, 有机化学, 2008, 28, 1487.)
[6] Zou, K. B.; Qiu, R. H.; Fang, D. W.; Liu, X. Y.; Xu, X. H. Synth. Commun. 2008, 38, 237.
[7] Zou, K. B.; Yin, X. H.; Liu, W. Q.; Qiu, R. H.; Li, L. X.; Shao, L. L.; Li, Y. H.; Xu, X. H.; Yang, R. H. Synth. Commun. 2009, 39, 646.
[8] Xia, X.; Zou, K. B.; Li, N. X.; Zhang, Z. Y.; Shao, L. L.; Xu, X. H. Acta Chim. Sinica 2008, 66, 1749. (夏湘, 邹康兵, 李诺信, 张尊英, 邵玲玲, 许新华, 化学学报, 2008, 66, 1749.)
[9] Steven, C. W.; Rayman, Y. W. Tetrahedron Lett. 1972, 13, 1853.
[10] Douglas, J. R.; Patrick, G.; Albert, O. B. J. Org. Chem. 1979, 44, 1149.
[11] Zaher, M. A. J.; Hao, Y. S.; Bun, C. C. Tetrahedron Lett. 2002, 43, 9381.
[12] Cheng, B. C.; Xie, W. L. J. Central South Univ. Technol. 1998, 29, 401 (in Chinese). (成本诚, 谢文林, 中南工业大学学报, 1998, 29, 401.)
[13] Chen, D. M.; Yang, H. R.; Li, B. N. Petrochem. Technol. 1999, 28, 528 (in Chinese). (陈达美, 杨辉荣, 黎碧娜, 石油化工, 1999, 28, 5.)
[14] Barratt, B. J. W.; Easton, C. J.; Simpson, J. S. PCT Int. Appl. 2004, 47, 87.
[15] Nielsen, N. M.; Bundgaard, H. Int. J. Pharm. 1987, 391, 75.
[16] Murai, K.; Akazome, G. Kogyo Kagaku Zasshi 1959, 62, 1094.
[17] Liu, Y.; Wei, M.; Liu, Z.-D. China Food Additives 2011, 4, 8.
[18] Sadieva, N. F.; Iskenderova, S. A.; Agaev, B. K.; Magerramova, S. N. Azerb. Khim. Zh. 2010, (4), 68.
[19] Nudelman, N. S.; Mendiara, S. Tetrahedron Lett. 1997, 38(13), 2245.
[20] Long, L.-P.; Zhong, T.-S.; Ding, L.-Z.; Yu, S.-X. Chin. J. Synth. Chem. 2002, 10(6), 542.
[21] Vijayakumar, B.; Nagendrappa, G.; Prakash, B. S. J. Indian J. Chem. Technol. 2009, 16(5), 377.
[22] Zhang, M.; Hanson, P. R. Sci. Synth. 2006, 20, 863.
/
| 〈 |
|
〉 |