

Chinese Journal of Organic Chemistry >
Study on the Regioselectivie of Grignard Reagent Addition Reaction of Maleopimaric Acid Trimethyl Ester
Received date: 2012-08-08
Revised date: 2012-09-25
Online published: 2012-09-26
Supported by
Project supported by the Project 973 (No. 2011CB512005), the National Natural Science Foundation of China (Nos. 81260472, 21101035), the Natural Science Foundation of Guangxi Province (No. 2011GXNSFD018010) and the Project of Ten, Hundred, Thousand Distinguished Talents in New Century of Guangxi Province (No. 2007228).
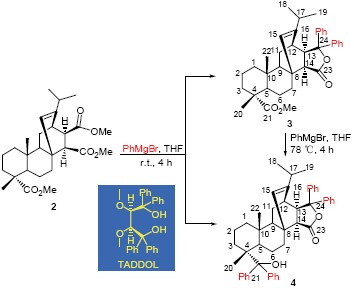
Two regioselectivite adducts (compounds 3 and 4) were firstly synthesized from the Grignard reagent and maleopimaric acid trimethyl ester (2). The structures of all compounds were characterized by elemental analyses, NMR and MS, and stero-structures of 3 were further determined by COSY, HMQC and HMBC. The spatial structure of 3 was further confirmed by X-ray single crystal diffraction analysis method. Owing to the addition reaction selectivity of 2 was dependent on the steric hindrance of three methyl groups, the TADDOL (tetraaryl-1,3-dioxolane-4,5-dimethanols) product was prevented because of the formation of lactone 3, but the stereo-structure of the rosin-ring did not changed. Products of 3 and 4 are expected to apply as chiral derivatization reagents.
Yao Guiyang , Wei Jingchen , Dai Weilong , Yang Da , Pan Yingming , Wang Hengshan . Study on the Regioselectivie of Grignard Reagent Addition Reaction of Maleopimaric Acid Trimethyl Ester[J]. Chinese Journal of Organic Chemistry, 2013 , 33(01) : 138 -142 . DOI: 10.6023/cjoc201208006
[1] Thomas, J. W.; Jolene, E. T. J. Org. Chem. 2000, 65, 5.
[2] Ann, E. L.; Thomas, J. W. Org. Lett. 2006, 8, 2823.
[3] Joseph G. Chirality 2008, 20, 5.
[4] Jiang X. X.; Zhang, Y. F.; Chan A. S. C.; Wang, R. Org. Lett. 2008, 11, 153.
[5] Jiang, X. X.; Zhang, Y. F.; Liu, X.; Zhang, G.; Lai, L. H.; Wu, L. P.; Zhang, J. N.; Wang, R. J. Org. Chem. 2009, 74, 5562.
[6] Jiang, X. X.; Zhang, Y. F.; Wu, L. P.; Zhang, G.; Liu, X.; Zhang, H. L.; Fu, D.; Wang, R. Adv. Synth. Catal. 2009, 351, 2096.
[7] Jiang, X. X.; Zhang, G.; Fu, D.; Cao, Y. M.; Shen, F. F.; Wang, R. Org. Lett. 2010, 12, 1544.
[8] Ye, F. G.; Wang, H. S.; Huang, B. J.; Zhao, S. L. Electrophoresis 2010, 31, 1488.
[9] Wang, H. S.; Zhao, S. L.; He, M.; Zhao, Z. C.; Pan, Y. M.; Liang, Q. J. Sep. Sci. 2007, 30, 2748.
[10] Zhao, S. L.; Wang, H. S.; Pan, Y. M.; He, M.; Zhao, Z. C. J. Chromatogr. A 2007, 1145, 246.
[11] Zhao, S. L.; Wang, H. S.; Zhang, R. C.; Tang, L. D.; Liu, Y. M. Electrophoresis 2006, 27, 17.
[12] Wang, H. S.; Zhang, R. C.; Zhao, S. L.; Tang, L. D.; Pan, Y. M. Anal. Chim. Acta 2006, 560, 64.
[13] Wang, H. S.; Tian, X. Y.; Yang, D.; Pan, Y. M.; Wu, Q.; He, C. H. Tetrahedron: Asymmetry 2011, 22, 381.
[14] Nishi, H.; Kuwahara, Y. J. Biochem. Biophys. Methods 2001, 48, 89.
[15] Nishi, H.; Kuwahara, Y. J. Pharm. Biomed. 2002, 27, 577.
[16] Chen, J.; Du, Y.; Zhu, F.; Chen, B. J. Chromatogr. A 2010, 1217, 7158.
[17] Zaher, M.; Baussanne, I.; Ravelet, C.; Halder, S.; Haroun, M.; Fize, J.; Décout, J.-L.; Peyrin, E. J. Chromatogr. A 2008, 1185, 291.
[18] Wang, H. S.; He, C. H.; Pan, Y. M.; Yao, G. Y.; Wu, Q.; Deng, H. G. J. Inclusion Phenom. Macrocyclic Chem. 2011, 73, 177.
[19] Sheldrick, G. M. SHELXTL, Version 6.10, Bruker AXS Inc., Madison, Wisconsin, USA, 2000.
[20] Pan, Y. M.; Yang, L.; Wang, H. S.; Zhang, R. C.; Zhang, Y. Acta Crystallogr., Sect. E 2006, 62, o5701.
/
| 〈 |
|
〉 |