

Chinese Journal of Organic Chemistry >
Study on Total Synthesis of Four Natural Prenylated Flavonoids
Received date: 2012-10-13
Revised date: 2012-10-31
Online published: 2012-11-02
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21162021, 20962016), the Ningxia Natural Science Foundation (No. NZ1006), the Program for New Century Excellent Talents in University (No. NCET-09-0860) and the National Basic Research Program 973 of China (No. 2010CB534916).
A facile approach for the total synthesis of prenylated flavonoids, (?)-abyssinone-VI-4-O-methyl ether (1), (?)-abyssinone-IV-4'-O-methyl ether (2), (?)-abyssinone-V-4'-O-methyl ether (3) and (?)-sigmoidin E (4), has been described. The key intermediate 4-hydroxy-3,5-di-(3-methylbut-2-enyl)benzaldehyde (6) was synthesized that features regioselective prenylation of 4-hydroxybenzaldehyde and crystallizing with petroleum ether from the reaction mixture by freeze-out effect. All structures of new compounds were confirmed by IR, 1H NMR, MS and HRMS techniques.
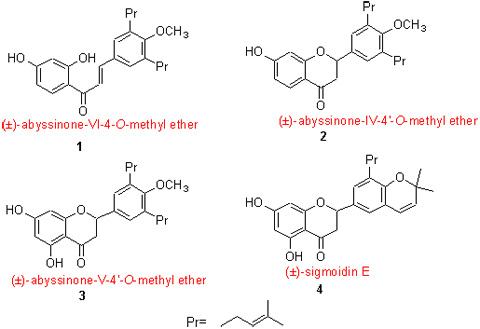
Zuo Wubiao , Yang Jinhui , Li Hongjun , Guo Dongdong , Luo Junshan , Huang Wenqian . Study on Total Synthesis of Four Natural Prenylated Flavonoids[J]. Chinese Journal of Organic Chemistry, 2012 , 32(12) : 2276 -2282 . DOI: 10.6023/cjoc201210016
[1] Salvatore, M. J.; Kng, A. B.; Graham A. C.; Onishi, H. R.; Bartizal, K. F.; Abruzzo, G. K.; Gill, C. J.; Ramjit, H. G.; Pitzenberger, S. M.; Witherup, K. M. J. Nat. Prod. 1998, 61, 640.
[2] Rahman, M. M.; Gray, A. I.; Khondkar, P.; Sarker, S. D. Pharm. Biol. 2008, 46, 356.
[3] Meragelman, T. L.; Tucker, K. D.; McClord, T. G.; Cardel-lina, J. H.; Shoemker, R. H. J. Nat. Prod. 2005, 68, 1790.
[4] Hirpara, K. V.; Aggarwal, P.; Mukherjee, A. J.; Joshi, N.; Burman, A. C. Curr. Med. Chem. 2009, 9, 138.
[5] Seshadri, T. R. Tetrahedron 1959, 6, 169.
[6] Yan, X.; Liu, H. Q.; Zou, Y. Q.; Ren, Z. H. Chin. J. Org. Chem. 2008, 28, 1534 (in Chinese).(延玺, 刘会青, 邹永青, 任占华, 有机化学, 2008, 28, 1534.)
[7] Oliver-Bever, B. Medicinal Plants in Tropical West Africa, Cambridge University Press, New York, 1981, p. 100.
[8] Na, M.; Jang, J.; Njamen, D.; Mbafor, J. T.; Fomum, Z. T.; Kim, B. Y.; Oh, W. K.; Ahn, J. S. J. Nat. Prod. 2006, 69, 1572.
[9] Moriyasu, M.; Ichimaru, M.; Nishiyama, Y.; Kato, A.; Mathenge, S. G; Juma, F. D.; Nganga, J. N. J. Nat. Prod. 1998, 61, 185.
[10] Yenesew, A.; Midiwo, J. O.; Miessner, M.; Heydenreich, M.; Peter, M. G. Phytochemistry 1998, 48, 1439.
[11] Promsattha, R.; Tempesta, M. S.; Fomum, Z. T.; Mbafor, J. T. J. Nat. Prod. 1988, 51, 611.
[12] Zhang, Y. H.; Yang, J. H.; Li, H. J.; Jiang, S. Z.; Li, Y. F.; Liu, W. Y. Chin. J. Chem. 2011, 29, 521.
[13] Yang, J. H.; Li, H. J.; Zhang, Y. H.; Jiang, S. Z.; Li, Y. F.; Xue, P.; Ma, Y. L.; Liu, W. Y. Chin. J. Org. Chem. 2011, 31, 1230 (in Chinese).(杨金会, 李红俊, 张玉恒, 江世智, 李云峰, 薛屏, 马玉龙, 刘万毅, 有机化学, 2011, 31, 1230.)
[14] Yang, J. H.; Luo, J. S.; Guo, D. D.; Huang, W. Q. Chin. J. Org. Chem. 2012, 32, 1749 (in Chinese).(杨金会, 落俊山, 郭冬冬, 黄文倩, 有机化学, 2012, 32, 1749.)
[15] Farmer, R. L.; Biddle, M. M.; Nibbs, A. E.; Huang, X. K.; Bergan, R. C.; Scheidt, K. A. ACS Med. Chem. Lett. 2010, 1, 400.
[16] Kazuaki, k.; Katsuo, H.; Sadakazu, Y.; Teruya, S.; Ichiro, T. S. Agric. Biol. Chem. 1975, 39, 133.
/
| 〈 |
|
〉 |