

Chinese Journal of Organic Chemistry >
Synthesis of 6-Trimethylsilyl-α-pyrone from 2,6-Bis(trimethylsilyl)- pyran via Mild Aerobic Oxidation
Received date: 2012-09-19
Revised date: 2012-10-24
Online published: 2012-11-15
Supported by
Project supported by the Doctoral Fund of Henan Polytechnic University (No. 648536).
In this study, an effective and mild method for the synthesis of 6-trimethylsilyl-α-pyrone was successfully developed. 6-Trimethylsilyl-α-pyrone, a very important intermediate in organic synthesis, was mildly synthesized from pyrane derivative via an aerobic oxidation at room temperature, without any solvents and catalysts. Furthermore, the possible mechanism of the reaction was proposed.
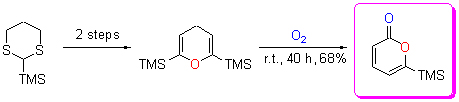
Key words: 6-trimethylsilyl-α-pyrone; oxidation; mechanism
Zhou Dejun , Yuji Matsuya . Synthesis of 6-Trimethylsilyl-α-pyrone from 2,6-Bis(trimethylsilyl)- pyran via Mild Aerobic Oxidation[J]. Chinese Journal of Organic Chemistry, 2013 , 33(02) : 375 -377 . DOI: 10.6023/cjoc201209027
[1] Tang, H.-Y.; Zeng, Y.; Li, Y.-Y.; Chen, J.-P.; Li, Y. Acta Chim. Sinica 2011, 69, 2241 (in Chinese). (唐海云, 曾毅, 李迎迎, 陈金平, 李嫕, 化学学报, 2011, 69, 2241.)
[2] Deng, Y.; Balunas, M. J.; Kim, J. A.; Lantvit, D. D.; Chin, Y. W.; Chin, H.; Sugiarso, S.; Kardono, L. B. S.; Fong, H. H. S.; Penzzuto, J. M.; Swanson, S. M.; Blanco, E. J. C.; Kinghorm, A. D. J. Nat. Prod. 2009, 72, 1165.
[3] Wang, H.; Wang, Y.; Wang, W.; Fu, P.; Liu, P.; Zhu, W. M. J. Nat. Prod. 2011, 74, 2014.
[4] Breda, S.; Reva, I.; Lapinski, L.; Cristiano, M. L. S.; Fausto, R. J. Phys. Chem. A 2006, 110, 6415.
[5] Sunazuka, T.; Omura, S. Chem. Rev. 2005, 105, 4559.
[6] Romero, D. L.; Manninen, P. R.; Han, F.; Romero, A. G. J. Org. Chem. 1999, 64, 4980.
[7] Tosaki, S. Y.; Nemoto, T.; Ohshima, T.; Shibasaki, M. Org. Lett. 2003, 5, 495.
[8] Peng, D.-Q.; Liu, Y.; Lv, Z.-F.; Xu, J.-H. Chin. J. Org. Chem. 2009, 29, 716 (in Chinese). (彭大权, 刘蕴, 吕志锋, 徐建华, 有机化学, 2009, 29, 716.)
[9] Cho, C. G.; Kim, Y. W.; Lim, Y. K.; Park, J. S.; Lee, H.; Koo, S. J. Org. Chem. 2002, 67, 290.
[10] Lanari, D.; Ballini, R.; Palmieri, A.; Pizzo, F.; Vaccaro, L. Eur. J. Org. Chem. 2011, 2874.
[11] Ohkata, K.; Lee, Y. G.; Utsumi, Y.; Ishimaru, K.; Akiba, K. Y. J. Org. Chem. 1991, 56, 5052.
[12] Perkin, W. H. J. Chem. Soc. 1868, 21, 181-186.
[13] Holden, M. S.; Crouch, R. D. J. Chem. Educ. 1998, 75, 1631.
[14] Larock, R. C.; Han, X. J.; Doty, M. J. Tetrahedron Lett. 1998, 39, 5713.
[15] Kotora, M.; Ishikawa, M.; Tsai, F. Y.; Takahashi, T. Tetrahedron 1999, 55, 4969
[16] Larock, R. C.; Doty, M. J.; Han X. J. J Org. Chem. 1999, 64, 8770.
[17] Chatadaj, W.; Corbet, M.; Furstnerm, A. Angew. Chem., Int. Ed. 2012, 51, 6929.
[18] Tsuda, T.; Morikawa, S.; Hasegawa, N.; Saegusa, T. J Org. Chem., 1990, 55, 2978.
[19] Huang, Q.; Campo, M. A.; Yao, T.; Tian, Q.; Larock, R. C. J. Org. Chem. 2004, 69, 8251.
[20] Yao, T.; Campo, M. A.; Larock, R. C. Org. Lett. 2004, 6, 2677.
[21] Saleur, D.; Bouillon, J. P.; Portella, C.; Hoffmann, N. Tetrahedron Lett. 2000, 41, 5199.
[22] Bouillon, J. P.; Portella, C. Eur. J. Org. Chem. 1999, 1571.
[23] Chuang, T. H.; Fang, J. M.; Jiaang, W. T.; Tsai, Y. M. J. Org. Chem. 1996, 61, 1794.
/
| 〈 |
|
〉 |