

Chinese Journal of Organic Chemistry >
A Facile and Efficient Synthesis of 2-Amino-6-methyl-4-aryltetrahydronaphthalene Derivatives
Received date: 2012-10-12
Revised date: 2012-11-21
Online published: 2012-11-26
Supported by
Project supported by the Natural Science Foundation of Jiangsu Education Department (No. 08KJB150017), the Foundation of Xuzhou Normal University (No. 10XLS02), and the Foundation from National Natural Science Foundation of China for Innovative Research Group (No. 50921002).
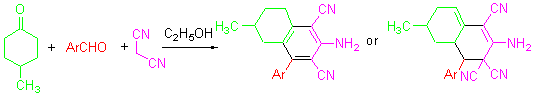
A facile and efficient synthesis of novel 2-amino-6-methyl-4-aryltetrahydronaphthalene derivatives by the reaction of aromatic aldehydes, 4-methylcyclohexanone, and malononitrile in the presence of NaOH and 95% EtOH is reported. This protocol has the advantages of shorter reaction time, mild conditions, and easy work-up. The products were identified by 1H NMR, IR and HRMS techniques. The reported method is the efficient approach for the synthesis of these compounds.
Rong Liangce , Wei Xianyong , Zong Zhimin , Han Xiao , Zhang Chengyi , Shen Xiaofeng , Chen Xiang . A Facile and Efficient Synthesis of 2-Amino-6-methyl-4-aryltetrahydronaphthalene Derivatives[J]. Chinese Journal of Organic Chemistry, 2013 , 33(03) : 648 -651 . DOI: 10.6023/cjoc201210014
/
| 〈 |
|
〉 |