

Chinese Journal of Organic Chemistry >
Preparation of Active Gnetol Dimers by Oxidative Coupling Reaction and Acid-Catalyzed Dimerization
Received date: 2012-10-29
Revised date: 2012-11-17
Online published: 2012-11-26
Oxidative coupling reaction with FeCl3?6H2O as oxidant and acid-catalyzed dimerization of natural gnetol in methanol afforded two new gnetol dimers and one new phenyl naphthalene derivative: 4-[1-(2,6-dihydroxyphenyl)-2-(3,5- dihydroxyphenyl)ethyl]-2-[(1E)-2-(3,5-dihydroxyphenyl)ethenyl]-1,3-benzenediol (1), 2-[1-(2,6-dihydroxyphenyl)-2-(3,5-di- hydroxyphenyl)ethyl]-5-[(1E)-2-(2,6-dihydroxyphenyl)ethenyl]-1,3-benzenediol (2) and 4-(6,8-dimethoxyl-2-naphthalenyl)- 1,3-benzenediol (3). Their structures were elucidated on the basis of spectral analysis, and their possible formation mechanisms were discussed. 1 and 2 were new linear stilbene dimers synthesized for the first time. Pharmacological tests showed 1, 2 and 3 to exhibit potent anti-oxidation activity with IC50 values of 6.29?10-9, 4.19?10-6, and 2.96?10-5 mol稬-1, respectively, and 2 was shown to have potent anti-inflammatory activity.
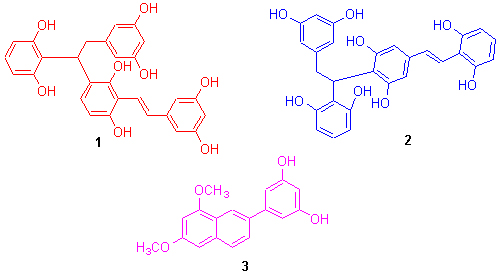
Key words: gnetol dimer; oxidative coupling reaction; bioactivity
Yao Chunsuo , Lin Mao , Yang Qingyun . Preparation of Active Gnetol Dimers by Oxidative Coupling Reaction and Acid-Catalyzed Dimerization[J]. Chinese Journal of Organic Chemistry, 2013 , 33(02) : 312 -318 . DOI: 10.6023/cjoc201210051
[1] Shimizu, K.; Kondo, R.; Sakai, K. Planta Med. 2000, 66, 11.
[2] Xu, G.; Zhang, L. Y.; Chen, L. F.; Hu, C. Q. Acta Pharm. Sin. 1994, 29, 818.
[3] Li, N.; Li, X. M.; Huang, K. S.; Lin, M. Acta Pharm. Sin. 2001, 36, 944.
[4] Wang, Y. H.; Huang, K. S.; Lin, M. J. Asian Nat. Prod. Res. 2001, 3, 169.
[5] Zhou, L. X.; Lin, M. Acta Pharm. Sin. 2000, 35, 669.
[6] Yao, C. S.; Zhou, L. X.; Lin, M. Chem. Pharm. Bull. 2004, 52, 238.
[7] Li, X. M.; Huang, K. S.; Lin, M.; Zhou, L. X. Tetrahedron 2003, 59, 4405.
[8] Huang, K. S.; Lin, M.; Wang, Y. H. Chin. Chem. Lett. 1999, 10, 817.
[9] Zhou, L. X.; Lin, M. Chin. Chem. Lett. 2000, 11, 515.
[10] Likhitwitayawuid, K.; Sritularak, B. J. Nat. Prod. 2001, 64, 1457.
[11] Li, J.; Cheng, G. F.; Zhu, X. Y. Acta Pharm. Sin. 2000, 35, 335.
[12] Dai, S. J.; Ma, Z. B.; Wu, Y.; Chen, R. Y.; Yu, D. Q. Phytochemistry 2004, 65, 3135.
/
| 〈 |
|
〉 |