

Chinese Journal of Organic Chemistry >
Novel Naphthalene-Based Crown Ether: Synthsis, Crystal Structure and Its Complexation with Paraquat Derivatives
Received date: 2012-11-20
Revised date: 2012-12-04
Online published: 2012-12-10
Supported by
Project supported by the China Postdoctoral Science Foundation (No. 2011M500989) and the Excellent Yong Teachers Program of Zhejiang Province (No. 00511024).
A Novel naphthalene-based crown ether has been synthesized by the etherification of 2,7-dihydroxynaphthalene with 8-tosyloxy-3,6-dioxaoctanol in the presence of K2CO3, followed by reaction with p-toluenesufonyl chloride to give 7. Compound 7 was further reacted with 2,7-dihydroxynaphthalene in the presence of cesium carbonate to give 1 in 38% total yield. Its crystal structure was determined by X-ray diffraction method. The crystal is of triclinic, space group P-1 with a= 8.165(3) Å, b=13.435(4) Å, c=14.083(3) Å, α=64.11(2)°, β=80.40(3)°, γ=88.19(3)°, V=1368.9(7) Å3, Z=2, Dc=1.331 g/cm3, λ=0.071070 nm, μ(Mo Kα)=1.331 mm-1, Mr=353.22, F(000)=584. The crystal structure of 1 shows that two napthalene units are linked two tri(ethylene glycol) bridges to form dinapthalzo[30]crown-8 cavity and one Z-like central cavity with size of ca. 8.2 Å×12.0 Å. The host 4 was proved to be a efficient host for the complexation with paraquat derivatives in solution by 1H NMR.
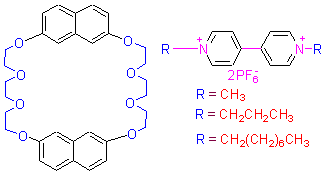
Key words: crown ethers; synthesis; crystal structure; complexation; paraquat derivatives
Zong Qianshou , Wu Jianyi . Novel Naphthalene-Based Crown Ether: Synthsis, Crystal Structure and Its Complexation with Paraquat Derivatives[J]. Chinese Journal of Organic Chemistry, 2013 , 33(03) : 568 -572 . DOI: 10.6023/cjoc201211037
/
| 〈 |
|
〉 |