

Chinese Journal of Organic Chemistry >
Synthesis of Novel Phenylacetylene Dendrimers Containing Different Protective Groups for Their Terminal Acetylenes
Received date: 2012-11-15
Revised date: 2012-12-14
Online published: 2012-12-18
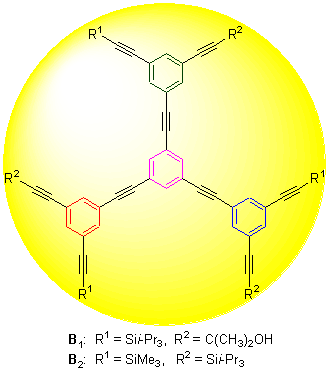
Using Sonogashira coupling reaction, two kinds of 1,3,5-substituted phenylacetylene dendrimers: 1,3,5-tris[3-(3- hydroxy-3-methyl-1-butynyl)-5-(triisopropylsilylethynyl)phenylethynyl]benzene (B1) and 1,3,5-tris[3-(trimethylsilylethynyl)- 5-(triisopropylsilylethynyl)phenylethynyl]benzene (B2) were synthetized and the synthetic routes were compared and discussed. The structures of the target compounds and intermediates were confirmed by 1H NMR, 13C NMR, EI-MS, HRMS techniques and element analysis. In phenylacetylene dendrimers B1 and B2, the six terminal acetylene groups on their periphery were protected by two different kinds of groups, respectively. Based on the different deprotection conditions, two different kinds of functional groups could be introduced to B1 and B2, respectively.
Xu Chen , Huang Pengcheng . Synthesis of Novel Phenylacetylene Dendrimers Containing Different Protective Groups for Their Terminal Acetylenes[J]. Chinese Journal of Organic Chemistry, 2013 , 33(03) : 551 -557 . DOI: 10.6023/cjoc201211031
/
| 〈 |
|
〉 |