

Chinese Journal of Organic Chemistry >
Synthesis and Antifungal Activity of Novel 1-(3-Indoly)-3-aryl-2-propen-1-one Oxime Ethers
Received date: 2012-12-16
Revised date: 2013-01-20
Online published: 2013-01-23
Supported by
Project supported by the Natural Science Foundation of Shandong Province (No. ZR2009BM044).
With the aim of finding novel indole derivatives with high antifungal activity, 3-acetyl indole was prepared using indole and other chemicals as starting materials through the Vilsmeier reaction, followed by reactions of aldol condensation and addition-elimination to synthesize a series of novel 2-propen-1-one oxime ethers containing indole moiety 3a~3j, and their structures were elucidated by IR, 1H NMR, MS techniques and elemental analysis. The target compounds were tested for in-vitro antifungal activities against Botrytis cinerea and Anthracnose pathogen by the mycelium growth rate method, and the result indicated that 3f and 3g displayed good antifungal activity against Botrytis cinerea at a concentration of 100 μg/mL, with inhibition rates of 81% and 74%, respectively.
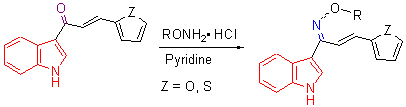
Key words: indole; propenone; oxime ether; synthesis; antifungal activity
Wang Meiyan , Qu Zhiqiang , Du Dan , Jiang Lin . Synthesis and Antifungal Activity of Novel 1-(3-Indoly)-3-aryl-2-propen-1-one Oxime Ethers[J]. Chinese Journal of Organic Chemistry, 2013 , 33(05) : 1005 -1009 . DOI: 10.6023/cjoc201212025
/
| 〈 |
|
〉 |