

Chinese Journal of Organic Chemistry >
Intramolecular Oxidative Coupling: Applications in Synthesis of Complex Indole Akaloid Scaffolds
Received date: 2013-01-15
Revised date: 2013-01-31
Online published: 2013-02-01
Supported by
Project supported by the National Basic Research Program of China (973 Program, No. 2010CB833200).
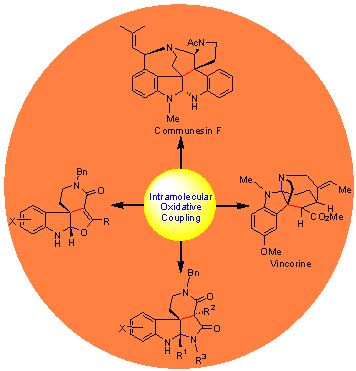
Intramolecular oxidative coupling of tryptamine incorporated amide was used to create the quaternary spiroindoline carbon center of communesins, which enabled a short asymmetric synthesis of (-)-communesins A and B and F. The fused tetra-ring framework of Vincorine was established by an intramolecular oxidative coupling/condensative cyclization process, which was further advanced to (-)-vincorine in 5 steps. From a medicinal standpoint, such a cascade process provides a highly diverse, efficient method for the construction of polycyclic spiroindoline scaffolds. Starting from easily accessible tryptamine incorporated β-ketoamides and malonamides, polyclic spiroindolines and pyrroloindolines could be directly obtained by adopting the same cascade strategy.
Xie Weiqing , Zuo Zhiwei , Zi Weiwei , Ma Dawei . Intramolecular Oxidative Coupling: Applications in Synthesis of Complex Indole Akaloid Scaffolds[J]. Chinese Journal of Organic Chemistry, 2013 , 33(05) : 869 -876 . DOI: 10.6023/cjoc201301035
[1] Selected examples for oxidative homo-coupling: (a) Ivanoff, D.; Spassoff, A. Bull. Soc. Chim. Fr. 1935, 2, 76.
(b) Rathke, M. W.; Lindert, A. J. Am. Chem. Soc. 1971, 93, 4605.
(c) Brocksom, T. J.; Petragnani, N.; Rodrigues, R.; La Scala Teixeira, H. Synthesis 1975, 396.
(d) Frazier, R. H.; Harlow, R. L. J. Org. Chem. 1980, 45, 5408.
(e) Belletire, J. L.; Fry, D. F. J. Org. Chem. 1987, 45, 2549.
(f) Renaud, P.; Fox, M. A. J. Org. Chem. 1988, 45, 3745.
(g) Quermann, R.; Maletz, R.; Schafer, H. J. Liegigs Ann. Chem. 1993, 11, 1219.
(h) Kise, N.; Tokioka, K.; Aoyama, Y. J. Org. Chem. 1995, 60, 1100.
(i) Langer, T.; Illich, M.; Felmchen, G. Tetrahedron Lett. 1995, 36, 4409.
(j) Kim, J. W.; Lee, J. J.; Lee, S.-H.; Ahn, K.-H. Synth. Commun. 1998, 28, 1287.
[2] Selected examples for oxidative hetero-coupling: (a) Ito, Y.; Konoike, T.; Harada, T.; Saegusa, T. J. Am. Chem. Soc. 1977, 99, 1487.
(b) Baran, P. S.; Richter, J. M.; Lin, D. W. Angew. Chem., Int. Ed. 2005, 44, 609.
(c) Baran, P. S.; DeMartino, M. P. Angew. Chem., Int. Ed. 2006, 45, 7083.
(d) Richter, J. M.; Whitefield, B.; Maimone, T. J.; Lin, D. W.; Castroviejo, P.; Baran, P. S. J. Am. Chem. Soc. 2007, 129, 12857.
(e) Richter, J. M.; Whitefield, B.; Maimone, T. J.; Lin, D. W.; Castroviejo, P.; Baran, P. S. J. Am. Chem. Soc. 2007, 129, 12857.
(f) DeMartino M. P.; Chen, K.; Baran, P. S. J. Am. Chem. Soc. 2008, 130, 11546.
[3] (a) Baran, P. S.; Richter, J. M. J. Am. Chem. Soc. 2004, 126, 7450.
(b) Baran, P. S.; Richter, J. M. J. Am. Chem. Soc. 2005, 127, 15394.
(c) Richter, J. M.; Whitefield, B.; Maimone, T. J.; Lin, D. W.; Castroviejo, P.; Baran, P. S. J. Am. Chem. Soc. 2007, 129, 12857.
(d) Baran, P. S.; Maimone, T. J.; Richter, J. M. Nature 2007, 446, 404.
(e) Richter, J. M.; Ishihara, Y.; Masuda, T.; Whitefield, B. W.; Llamas, T.; Pohjakallio, A.; Baran, P. S. J. Am. Chem. Soc. 2008, 130, 17938.
[4] (a) Baran, P. S.; Richter, J. M. J. Am. Chem. Soc. 2004, 126, 7450.
(b) Baran, P. S.; Richter, J. M. J. Am. Chem. Soc. 2005, 127, 15394.
(c) Baran, P. S.; Maimone, T. J.; Richter, J. M. Nature 2007, 446, 404.
(d) Richter, J. M.; Ishihara, Y.; Masuda, T.; Whitefield, B. W.; Llamas, T.; Pohjakallio, A.; Baran, P. S. J. Am. Chem. Soc. 2008, 130, 17938.
[5] (a) Martin, C. L.; Overman, L. E.; Rohde, J. A. J. Am. Chem. Soc. 2010, 132, 4894.
(b) Martin, C. L.; Overman, L. E.; Rohde, J. A. J. Am. Chem. Soc. 2008, 130, 7568.
Fore review see: (c) Guo, F.; Clift, M. D.; Thomson, R. J. Eur. J. Org. Chem. 2012, 26, 4881.
For other examples see: (d) Li, Q.; Fan, A.; Lu, Z.; Cui, Y.; Lin, W.; Jia, Y. Org. Lett. 2010, 12, 4066.
(e) Li, Q.; Jiang, J.; Fan, A.; Cui, Y.; Jia, Y. Org. Lett. 2011, 13, 312.
[6] (a) Zuo, Z.; Xie, W.; Ma, D. J. Am. Chem. Soc. 2010, 132, 13226.
(b) Zuo, Z.; Ma, D. Angew. Chem., Int. Ed. 2011, 50, 12008.
[7] Zi, W.; Xie, W.; Ma, D. J. Am. Chem. Soc. 2012, 132, 9126.
[8] Fan, F.; Xie, W.; Ma, D. Org. Lett. 2012, 14, 1405.
[9] Fan, F.; Xie, W.; Ma, D. Chem. Commun. 2012, 48, 7571.
[10] (a) Numata, C.; Takahashi, Y.; Ito, T.; Takada, K.; Kawai, Y.; Usami, E.; Matsumura, M.; Imachi, T.; Ito, T.; Hasegawa, T. Tetrahedron Lett. 1993, 34, 2355.
(b) Jadulco, R.; Edrada, R. A.; Ebel, R.; Berg, A.; Schauman, K.; Wray, V.; Steube, K.; Proksch, P. J. Nat. Prod. 2004, 67, 78.
(c) Hayashi, H.; Matsumoto, H.; Akiyama, K. Biosci. Biotechnol. Biochem. 2004, 68, 753.
(d) Dalsgaard, P. W.; Blunt, J. W.; Munro, M. H. G.; Frisvad, J. C.; Christophersen, C. J. Nat. Prod. 2005, 68, 258.
[11] For synthetic studies see: (a) May, J. A.; Stoltz, B. M. Tetrahedron 2006, 62, 5262.
(b) Crawley, S. L.; Funk, R. L. Org. Lett. 2006, 8, 3995.
(c) George, J. H.; Adlington, R. M. Synlett 2008, 14, 2093.
For total syntheses see Refs. [6] and (d) Yang, J.; Wu, H.; Shen, L.; Qin, Y. J. Am. Chem. Soc. 2007, 129, 13794.
(e) Liu, P.; Seo, J. H.; Weinreb, S. M. Angew. Chem. 2010, 122, 2044; Angew. Chem., Int. Ed. 2010, 49, 2000.
(f) Belmar, J.; Funk, R. L. J. Am. Chem. Soc. 2012, 134, 16941.
[12] (a) Smith, G. F. Chem. Ind. 1961, 1120.
(b) Wenkert, E.; Wickberg, B. J. Am. Chem. Soc. 1965, 87, 1580.
For an overview of the akuammiline alkaloid family, see: (c) Ramirez, A.; Garcia-Rubio, S. Curr. Med. Chem. 2003, 10, 1891.
[13] (a) Mokry, J.; Dubravkova, L.; Sefcovic, P. Experientia 1962, 18, 564.
(b) Das, B. C.; Cosson, J. P.; Lukacs, G.; Potier, P. Tetrahedron Lett. 1974, 15, 4229.
(c) Mamatas-Kalamaras, S.; Sevenet, T.; Thal, C.; Potier, P. Phytochemistry 1975, 14, 1637.
[14] Subramaniam, G.; Hiraku, O.; Hayashi, M.; Koyano, T.; Komiyama, K.; Kam, T. S. J. Nat. Prod. 2007, 70, 1783.
[15] (a) Schnoes, H.; Biemann, K.; Mokry, J.; Kompis, I.; Chatterjee, A.; Ganguli, G. J. Org. Chem. 1966, 31, 1641.
(b) Ahmad, Y.; Fatima, K.; Rahman, A.; Occolowitz, J.; Solheim, B.; Clardy, J.; Garnick, R.; Le Quesne, P. J. Am. Chem. Soc. 1977, 99, 1943.
[16] Cai, X. H.; Tan, Q. G.; Liu, Y. P.; Feng, T.; Du, Z. Z.; Li, W. Q.; Luo, X. D. Org. Lett. 2008, 10, 577.
[17] (a) Dounay, D. B.; Vollhardt, K. P. C. J. Am. Chem. Soc. 1990, 112, 5653.
(b) Lévy, J.; Sapi, J.; Laronze, J. Y.; Royer, D.; Toupet, L. Synlett 1992, 7, 601.
(c) Dounay, A. B.; Overman, L. E.; Wrobleski, A. D. J. Am. Chem. Soc. 2005, 127, 10186.
(d) Shen, L.; Zhang, M.; Wu, Y.; Qin, Y. Angew. Chem., Int. Ed. 2008, 47, 3618.
(e) Jones, S. B.; Simons, B.; Macmillan, D. W. C. J. Am. Chem. Soc. 2009, 131, 13606.
(f) Yasui, Y.; Kinugawab, T.; Takemoto, Y. Chem. Commun. 2009, 28, 4275.
(g) Zhang, M.; Huang, X.; Shen, L.; Qin, Y. J. Am. Chem. Soc. 2009, 131, 6013.
(h) Zhang, D.; Song, H.; Qin, Y. Acc. Chem. Res. 2011, 44, 447.
(i) Zu, L.; Boal, B. W.; Garg, N. K. J. Am. Chem. Soc. 2011, 133, 8877.
(j) Adams, G. L.; Caroll, P. J.; Smith, A. B. J. Am. Chem. Soc. 2012, 134, 4037.
[18] For reviews, see: (a) Anthoni, U.; Christophersen, C.; Nielsen, P. H. In Alkaloids: Chemical and Biological Perspectives, Vol. 13, Ed.: Pelletier, S. W., Pergamon, New York, 1999, p. 163.
(b) Cordell, G. A.; Saxton, J. E. In the Alkaloids: Chemistry and Physiology, Vol. 20, Eds.: Manske, R. H. F.; Rodrigo, R. G. A., Academic Press, New York, 1981, p. 3.
(c) Hino, T.; Nakagawa, M. In the Alkaloids: Chemistry and Pharmacology, Vol. 34, Ed.: Brossi, A., Academic Press, New York, 1989, p. 1.
(d) Sévenet, T.; Pusset, J. In the Alkaloids: Chemistry and Pharmacology, Vol. 48, Ed.: Cordell, G. A., Academic Press, New York, 1996, p. 1.
(e) Steven, A.; Overman, L. E. Angew. Chem., Int. Ed. 2007, 46, 5488.
(f) Ruiz-Sanchis, P.; Savina, S. A.; Albericio, F.; Álvarez, M. Chem. Eur. J. 2011, 17, 1388.
/
| 〈 |
|
〉 |