

Chinese Journal of Organic Chemistry >
Synthesis of Chirl N,P-ligands Derived from L-Proline and Its Application to 1,3-Dipolar Cycloaddition
Received date: 2013-01-31
Revised date: 2013-02-26
Online published: 2013-03-01
Supported by
Project supported by the National Natural Science Foundation of China (No. 20902015) and the Fundamental Research Funds for the Central Universities (No. 2010055).
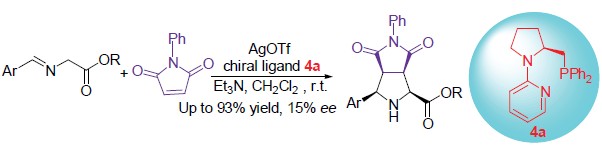
Aminophosphine 2 was obtained by deprotection of amidophosphine 1, which was derived from L-proline. Deprotonation of aminophosphine 2 with n-BuLi, and then being captured by 2-MeO-pyridine gave chiral pyridyl N,P-ligands 4. Reaction of aminoalcohol and diethyl carbonate afforded oxazolidione 6, which was converted to compounds 7 by the reaction with triethyloxonium tetrafluoroborate. Chiral N,P-ligands 8 were obtained by the reaction of compounds 7 with aminophosphine 2. Two kinds of chiral N,P-ligands shown excellent catalytic activity in Ag(I) catalytic asymmetric 1,3-dipolar cycloaddition, and up to 93% yield and 15% ee were obtained.
Chen Hong , Chen Qian . Synthesis of Chirl N,P-ligands Derived from L-Proline and Its Application to 1,3-Dipolar Cycloaddition[J]. Chinese Journal of Organic Chemistry, 2013 , 33(04) : 848 -853 . DOI: 10.6023/cjoc201301080
[1] Pandey, G.; Banerjee, P.; Gadre, S. R. Chem. Rev. 2006, 106, 4484.
[2] Reviews for 1, 3-dipolar cycloaddition reaction:
(a) Coldham, I.; Hufton, R. Chem. Rev. 2005, 105, 2765.
(b) Najera, C.; Sansano, J. M. Angew. Chem., Int. Ed. 2005, 44, 6272.
(c) 羖varez-Corral, M.; Muñoz-Dorado, M.; Rodr韌uez-Garc韆, I. Chem. Rev. 2008, 108, 3174.
(d) Stanley, L. M.; Sibi, M. P. Chem. Rev. 2008, 108, 2887;
(e) Engels, B.; Christl, M. Angew. Chem., Int. Ed. 2009, 48, 7968.
(f) Adrio, J.; Carretero, J. C. Chem. Commun. 2011, 47, 6784.
[3] (a) Longmire, J. M.; Wang, B.; Zhang, X. J. Am. Chem. Soc. 2002, 124, 13400.
(b) Gao, W.; Zhang, X.; Raghunath, M. Org. Lett. 2005, 7, 4241.
[4] (a) Gothelf, A. S.; Gothelf, K. V.; Hazell, R. G.; Jørgensen, K. A. Angew. Chem., Int. Ed. 2002, 41, 4236.
(b) Alemparte, C.; Blay, G.; Jørgensen, K. A. Org. Lett. 2005, 7, 4569.
[5] Chen, C.; Li, X.; Schreiber, S. L. J. Am. Chem. Soc. 2003, 125, 10174.
[6] (a) Cabrera, S.; Array醩, R. G.; Carretero, J. C. J. Am. Chem. Soc. 2005, 127, 16394.
(b) Cabrera, S.; Array醩, R. G.; Martin-Matute, B.; Cossio, F. P.; Carretero, J. C. Tetrahedron 2007, 63, 6587.
(c) Llamas, T.; Array醩, R. G.; Carretero, J. C. Org. Lett. 2006, 8, 1795.
(d) Llamas, T.; Array醩, R. G.; Carretero, J. C. Synthesis 2007, 950.
[7] (a) Zeng, W.; Chen, G.-Y.; Zhou, Y.-G.; Li, Y.-X. J. Am. Chem. Soc. 2007, 129, 750.
(b) Zeng, W.; Zhou, Y.-G. Org. Lett. 2005, 7, 5055.
(c) Zeng, W.; Zhou, Y.-G. Tetrahedron Lett. 2007, 48, 4619
[8] Yan, X.-X.; Peng, Q.; Zhang, Y.; Zhang, K.; Hong, W.; Hou, X.-L.; Wu, Y.-D. Angew. Chem., Int. Ed. 2006, 45, 1979.
[9] (a) N醞era, C.; De Gracia Retamosa, M.; Sansano, J. M. Org. Lett. 2007, 9, 4025.
(b) N醞era, C.; De Gracia Retamosa, M.; Sansano, J. M. Angew. Chem., Int. Ed. 2008, 47, 6055
[10] Wang, C.-J.; Liang, G.; Xue, Z.-Y.; Gao, F. J. Am. Chem. Soc. 2008, 130, 17250.
[11] Yamashita, Y.; Guo, X.-X.; Takashita, R.; Kobayashi, S. J. Am. Chem. Soc. 2010, 132, 3262.
[12] Shi, M.; Shi, J.-W. Tetrahedron: Asymmetry 2007, 18, 645.
[13] Arai, T., Mishiro, A.; Yokoyama, N.; Suzuki, K.; Sato, H. J. Am. Chem. Soc. 2010, 132, 5338.
[14] (a) Nakagawa, Y.; Kanai, M.; Nagaoka, Y.; Tomioka, K. Tetrahedron 1998, 54, 10295.
(b) Kanai, M.; Nakagawa, Y.; Tomioka, K. Tetrahedron 1999, 55, 3843.
(c) Nakagawa, Y.; Matsumoto, K.; Tomioka, K. Tetrahedron 2000, 56, 2857.Gawley, R. E.; Hart, G. C.; Bartolotti, L. J. J. Org. Chem. 1989, 54, 175.
/
| 〈 |
|
〉 |