

Chinese Journal of Organic Chemistry >
Synthesis and Biological Activity of 1-{4-[(2-Cyanoimino-1,3-thiazolidine-3-yl)methyl]thiazol-2-yl}-3-aroyl Ureas
Received date: 2013-01-29
Revised date: 2013-02-07
Online published: 2013-03-06
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21102077, 21202089), the Science and Technology Innovation Foundation for the College Students of China (No. 201210304039) and the Research Foundation of the Six People Peak of Jiangsu Province (No. 2011-SWYY-009).
In search of novel thiazole derivatives with potent biological activities, a series of new thiazole compounds 6 containing acyl urea moiety were synthesized by the condensation of 2-cyanoimino-3-(2-aminothiazol-4-ylmethyl)thiazolidine with various arylacylisocyanates. The structures of the target compounds were determined by 1H NMR, MS and elemental analysis. The structures of 6a, 6e and 6h were further characterized by 13C NMR spectra. The bioassay data indicated that some of the title compounds showed fungicidal activities to some extent at the concentration of 50 μg/mL. For example, compound 6d displayed 65.3% inhibition rate against Gibberella zeae, compound 6f exhibited 67.3% inhibition rate against Cercospora arachidicola, and compound 6g showed 56.1% inhibition rate against Physalospora piricola.
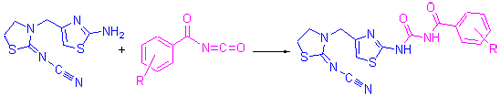
Key words: acyl urea; thiazole; synthesis; biological activity
Dai Hong , Zhao Yuanfei , Niu Ping , Qian Yijun , Li Yongqiang , Fang Jianxin , Shi Yujun . Synthesis and Biological Activity of 1-{4-[(2-Cyanoimino-1,3-thiazolidine-3-yl)methyl]thiazol-2-yl}-3-aroyl Ureas[J]. Chinese Journal of Organic Chemistry, 2013 , 33(07) : 1568 -1572 . DOI: 10.6023/cjoc201301068
/
| 〈 |
|
〉 |